Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory
- PMID: 17369425
- PMCID: PMC1914193
- DOI: 10.1104/pp.107.097154
Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory
Abstract
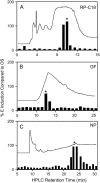
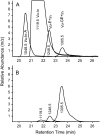
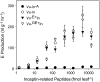
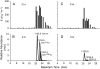
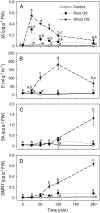
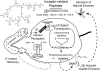
In cowpea (Vigna unguiculata), fall armyworm (Spodoptera frugiperda) herbivory and oral secretions (OS) elicit phytohormone production and volatile emission due to inceptin [Vu-In; (+)ICDINGVCVDA(-)], a peptide derived from chloroplastic ATP synthase gamma-subunit (cATPC) proteins. Elicitor-induced plant volatiles can function as attractants for natural enemies of insect herbivores. We hypothesized that inceptins are gut proteolysis products and that larval OS should contain a mixture of related peptides. In this study, we identified three additional cATPC fragments, namely Vu-(GE+)In [(+)GEICDINGVCVDA(-)], Vu-(E+)In [(+)EICDINGVCVDA(-)], and Vu-In(-A) [(+)ICDINGVCVD(-)]. Leaf bioassays for induced ethylene (E) production demonstrated similar effective concentration(50) values of 68, 45, and 87 fmol leaf(-1) for Vu-In, Vu-(E+)In, and Vu-(GE+)In, respectively; however, Vu-In(-A) proved inactive. Shortly following ingestion of recombinant proteins harboring cATPC sequences, larval OS revealed similar concentrations of the three elicitors with 80% of the potential inceptin-related peptides recovered. Rapidly shifting peptide ratios over time were consistent with continued proteolysis and preferential stability of inceptin. Likewise, larvae ingesting host plants with inceptin precursors containing an internal trypsin cleavage site rapidly lost OS-based elicitor activity. OS containing inceptin elicited a rapid and sequential induction of defense-related phytohormones jasmonic acid, E, and salicylic acid at 30, 120, and 240 min, respectively, and also the volatile (E)-4,8-dimethyl-1,3,7-nonatriene. Similar to established peptide signals such as systemin and flg22, amino acid substitutions of Vu-In demonstrate an essential role for aspartic acid residues and an unaltered C terminus. In cowpea, insect gut proteolysis following herbivory generates inappropriate fragments of an essential metabolic enzyme enabling plant non-self-recognition.
Figures

Similar articles
-
An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defenses.Plant Physiol. 2012 Nov;160(3):1468-78. doi: 10.1104/pp.112.201061. Epub 2012 Sep 24. Plant Physiol. 2012. PMID: 23008466 Free PMC article.
-
Fragments of ATP synthase mediate plant perception of insect attack.Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8894-9. doi: 10.1073/pnas.0602328103. Epub 2006 May 23. Proc Natl Acad Sci U S A. 2006. PMID: 16720701 Free PMC article.
-
The attraction of Spodoptera frugiperda neonates to cowpea seedlings is mediated by volatiles induced by conspecific herbivory and the elicitor inceptin.J Chem Ecol. 2008 Mar;34(3):291-300. doi: 10.1007/s10886-007-9414-y. Epub 2008 Feb 7. J Chem Ecol. 2008. PMID: 18256881
-
Synthetic elicitors-induced defense in crops against herbivory: A review.Plant Sci. 2025 Mar;352:112387. doi: 10.1016/j.plantsci.2025.112387. Epub 2025 Jan 8. Plant Sci. 2025. PMID: 39793711 Review.
-
Indirect plant defense against insect herbivores: a review.Insect Sci. 2018 Feb;25(1):2-23. doi: 10.1111/1744-7917.12436. Epub 2017 Mar 20. Insect Sci. 2018. PMID: 28035791 Review.
Cited by
-
Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling.Plant Physiol. 2009 Jul;150(3):1576-86. doi: 10.1104/pp.109.139550. Epub 2009 May 20. Plant Physiol. 2009. PMID: 19458114 Free PMC article.
-
A receptor-like protein mediates plant immune responses to herbivore-associated molecular patterns.Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):31510-31518. doi: 10.1073/pnas.2018415117. Epub 2020 Nov 23. Proc Natl Acad Sci U S A. 2020. PMID: 33229576 Free PMC article.
-
Understanding Plant Social Networking System: Avoiding Deleterious Microbiota but Calling Beneficials.Int J Mol Sci. 2021 Mar 24;22(7):3319. doi: 10.3390/ijms22073319. Int J Mol Sci. 2021. PMID: 33805032 Free PMC article. Review.
-
Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels.Cells. 2023 Jan 7;12(2):251. doi: 10.3390/cells12020251. Cells. 2023. PMID: 36672186 Free PMC article. Review.
-
Jasmonate signaling and manipulation by pathogens and insects.J Exp Bot. 2017 Mar 1;68(6):1371-1385. doi: 10.1093/jxb/erw478. J Exp Bot. 2017. PMID: 28069779 Free PMC article. Review.
References
-
- Alghali AM (1991) Integrated pest-management strategy for cowpea production under residual soil-moisture in the Bida area of Northern Nigeria. Trop Pest Manage 37 224–227
-
- Axtell MJ, Staskawicz BJ (2003) Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112 369–377 - PubMed
-
- Boland W, Hopke J, Donath J, Nuske J, Bublitz F (1995) Jasmonic acid and coronatin induce odor production in plants. Angew Chem Int Ed Engl 34 1600–1602
-
- Boller T (2005) Peptide signalling in plant development and self/non-self perception. Curr Opin Cell Biol 17 116–122 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

