Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover
- PMID: 17369430
- PMCID: PMC1913808
- DOI: 10.1104/pp.107.095794
Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover
Abstract
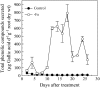
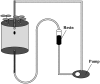
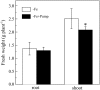
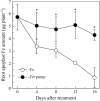
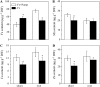
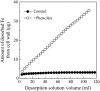
Phenolic compounds are frequently reported to be the main components of root exudates in response to iron (Fe) deficiency in Strategy I plants, but relatively little is known about their function. Here, we show that removal of secreted phenolics from the root-bathing solution almost completely inhibited the reutilization of apoplastic Fe in roots of red clover (Trifolium pratense). This resulted in much lower levels of shoot Fe and significantly higher root Fe compared with control and also resulted in leaf chlorosis, suggesting this approach stimulated Fe deficiency. This was supported by the observation that phenolic removal significantly enhanced root ferric chelate reductase activity, which is normally induced by plant Fe deficiency. Furthermore, root proton extrusion, which also is normally increased during Fe deficiency, was found to be higher in plants exposed to the phenolic removal treatment too. These results indicate that Fe deficiency-induced phenolics secretion plays an important role in the reutilization of root apoplastic Fe, and this reutilization is not mediated by proton extrusion or the root ferric chelate reductase. In vitro studies with extracted root cell walls further demonstrate that excreted phenolics efficiently desorbed a significant amount of Fe from cell walls, indicating a direct involvement of phenolics in Fe remobilization. All of these results constitute the first direct experimental evidence, to our knowledge, that Fe deficiency-induced secretion of phenolics by the roots of a dicot species improves plant Fe nutrition by enhancing reutilization of apoplastic Fe, thereby improving Fe nutrition in the shoot.
Figures


Similar articles
-
Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis.Plant Cell Environ. 2014 Apr;37(4):852-63. doi: 10.1111/pce.12203. Epub 2013 Nov 5. Plant Cell Environ. 2014. PMID: 24111973
-
Inhibition of shoot-expressed NRT1.1 improves reutilization of apoplastic iron under iron-deficient conditions.Plant J. 2022 Oct;112(2):549-564. doi: 10.1111/tpj.15967. Epub 2022 Sep 19. Plant J. 2022. PMID: 36062335
-
Red clover (Trifolium pratense L.) isoflavones: root phenolic compounds affected by biotic and abiotic stress factors.J Sci Food Agric. 2010 Feb;90(3):418-23. doi: 10.1002/jsfa.3831. J Sci Food Agric. 2010. PMID: 20355062
-
New routes for plant iron mining.New Phytol. 2017 Apr;214(2):521-525. doi: 10.1111/nph.14364. Epub 2016 Dec 5. New Phytol. 2017. PMID: 27918629 Review.
-
Towards a knowledge-based correction of iron chlorosis.Plant Physiol Biochem. 2011 May;49(5):471-82. doi: 10.1016/j.plaphy.2011.01.026. Epub 2011 Feb 3. Plant Physiol Biochem. 2011. PMID: 21349731 Review.
Cited by
-
Sodium nitroprusside-mediated alleviation of iron deficiency and modulation of antioxidant responses in maize plants.AoB Plants. 2010;2010:plq002. doi: 10.1093/aobpla/plq002. Epub 2010 Feb 15. AoB Plants. 2010. PMID: 22476060 Free PMC article.
-
Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion.Front Plant Sci. 2020 Jan 24;10:1741. doi: 10.3389/fpls.2019.01741. eCollection 2019. Front Plant Sci. 2020. PMID: 32038698 Free PMC article. Review.
-
Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis.J Exp Bot. 2018 Mar 24;69(7):1735-1748. doi: 10.1093/jxb/ery005. J Exp Bot. 2018. PMID: 29361149 Free PMC article.
-
Changes in Growth and Heavy Metal and Phenolic Compound Accumulation in Buddleja cordata Cell Suspension Culture under Cu, Fe, Mn, and Zn Enrichment.Plants (Basel). 2024 Apr 19;13(8):1147. doi: 10.3390/plants13081147. Plants (Basel). 2024. PMID: 38674556 Free PMC article.
-
The activation of iron deficiency responses of grapevine rootstocks is dependent to the availability of the nitrogen forms.BMC Plant Biol. 2024 Mar 26;24(1):218. doi: 10.1186/s12870-024-04906-y. BMC Plant Biol. 2024. PMID: 38532351 Free PMC article.
References
-
- Azia F, Stewart KA (2001) Relationships between extractable chlorophyll and SPAD values in muskmelon leaves. J Plant Nutr 24 961–966
-
- Blum U, Staman KL, Flint LJ, Shafer SR (2000) Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. J Chem Ecol 26 2059–2078
-
- Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
-
- Cao G, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 22 749–760 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

