Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport
- PMID: 17369433
- PMCID: PMC1913782
- DOI: 10.1104/pp.106.094748
Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport
Abstract
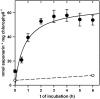
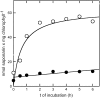
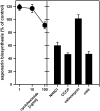
Barley (Hordeum vulgare) primary leaves synthesize saponarin, a 2-fold glucosylated flavone (apigenin 6-C-glucosyl-7-O-glucoside), which is efficiently accumulated in vacuoles via a transport mechanism driven by the proton gradient. Vacuoles isolated from mesophyll protoplasts of the plant line anthocyanin-less310 (ant310), which contains a mutation in the chalcone isomerase (CHI) gene that largely inhibits flavonoid biosynthesis, exhibit strongly reduced transport activity for saponarin and its precursor isovitexin (apigenin 6-C-glucoside). Incubation of ant310 primary leaf segments or isolated mesophyll protoplasts with naringenin, the product of the CHI reaction, restores saponarin biosynthesis almost completely, up to levels of the wild-type Ca33787. During reconstitution, saponarin accumulates to more than 90% in the vacuole. The capacity to synthesize saponarin from naringenin is strongly reduced in ant310 miniprotoplasts containing no central vacuole. Leaf segments and protoplasts from ant310 treated with naringenin showed strong reactivation of saponarin or isovitexin uptake by vacuoles, while the activity of the UDP-glucose:isovitexin 7-O-glucosyltransferase was not changed by this treatment. Our results demonstrate that efficient vacuolar flavonoid transport is linked to intact flavonoid biosynthesis in barley. Intact flavonoid biosynthesis exerts control over the activity of the vacuolar flavonoid/H(+)-antiporter. Thus, the barley ant310 mutant represents a novel model system to study the interplay between flavonoid biosynthesis and the vacuolar storage mechanism.
Figures




Similar articles
-
Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H(+)-antiport and ATP-binding cassette-type mechanisms.Plant Physiol. 2002 Feb;128(2):726-33. doi: 10.1104/pp.010590. Plant Physiol. 2002. PMID: 11842175 Free PMC article.
-
Changes in isovitexin-O-glycosylation during the development of young barley plants.Phytochemistry. 2018 Apr;148:11-20. doi: 10.1016/j.phytochem.2018.01.001. Epub 2018 Feb 6. Phytochemistry. 2018. PMID: 29421507
-
Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside.J Biol Chem. 1996 Nov 22;271(47):29666-71. doi: 10.1074/jbc.271.47.29666. J Biol Chem. 1996. PMID: 8939899
-
Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach.Plant Physiol. 2006 May;141(1):196-207. doi: 10.1104/pp.106.079533. Epub 2006 Mar 31. Plant Physiol. 2006. PMID: 16581873 Free PMC article.
-
Saponarin, a Di-glycosyl Flavone from Barley (Hordeum vulgare L.): An Effective Compound for Plant Defense and Therapeutic Application.ACS Omega. 2023 Jun 14;8(25):22285-22295. doi: 10.1021/acsomega.3c00267. eCollection 2023 Jun 27. ACS Omega. 2023. PMID: 37396229 Free PMC article. Review.
Cited by
-
A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota.Metabolites. 2019 Nov 9;9(11):272. doi: 10.3390/metabo9110272. Metabolites. 2019. PMID: 31717482 Free PMC article. Review.
-
Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties.Biomed Res Int. 2015;2015:905215. doi: 10.1155/2015/905215. Epub 2015 Feb 23. Biomed Res Int. 2015. PMID: 25802870 Free PMC article. Review.
-
Transcriptomic Analysis of Paeonia delavayi Wild Population Flowers to Identify Differentially Expressed Genes Involved in Purple-Red and Yellow Petal Pigmentation.PLoS One. 2015 Aug 12;10(8):e0135038. doi: 10.1371/journal.pone.0135038. eCollection 2015. PLoS One. 2015. PMID: 26267644 Free PMC article.
-
Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum).Plants (Basel). 2024 Jul 5;13(13):1861. doi: 10.3390/plants13131861. Plants (Basel). 2024. PMID: 38999705 Free PMC article.
-
Formulation of Herbal Gel of Antirrhinum majus Extract and Evaluation of its Anti-Propionibacterium acne Effects.Adv Biomed Res. 2018 Mar 27;7:53. doi: 10.4103/abr.abr_99_17. eCollection 2018. Adv Biomed Res. 2018. PMID: 29657938 Free PMC article.
References
-
- Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR (2003) The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J 35 624–636 - PubMed
-
- Amrhein N (1979) Biosynthesis of cyanidin in buckwheat hypocotyls. Phytochemistry 18 585–589
-
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102 2649–2654 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

