Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis
- PMID: 17369438
- PMCID: PMC1913769
- DOI: 10.1104/pp.106.092130
Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis
Abstract
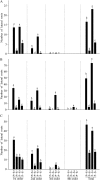
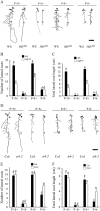
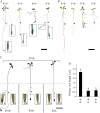
Phosphorus, one of the essential elements for plants, is often a limiting nutrient in soils. Low phosphate (Pi) availability induces sugar-dependent systemic expression of genes and modulates the root system architecture (RSA). Here, we present the differential effects of sucrose (Suc) and auxin on the Pi deficiency responses of the primary and lateral roots of Arabidopsis (Arabidopsis thaliana). Inhibition of primary root growth and loss of meristematic activity were evident in seedlings grown under Pi deficiency with or without Suc. Although auxin supplementation also inhibited primary root growth, loss of meristematic activity was observed specifically under Pi deficiency with or without Suc. The results suggested that Suc and auxin do not influence the mechanism involved in localized Pi sensing that regulates growth of the primary root and therefore delineates it from sugar-dependent systemic Pi starvation responses. However, the interaction between Pi and Suc was evident on the development of the lateral roots and root hairs in the seedlings grown under varying levels of Pi and Suc. Although the Pi+ Suc- condition suppressed lateral root development, induction of few laterals under the Pi- Suc- condition point to increased sensitivity of the roots to auxin during Pi deprivation. This was supported by expression analyses of DR5uidA, root basipetal transport assay of auxin, and RSA of the pgp19 mutant exhibiting reduced auxin transport. A significant increase in the number of lateral roots under the Pi- Suc- condition in the chalcone synthase mutant (tt4-2) indicated a potential role for flavonoids in auxin-mediated Pi deficiency-induced modulation of RSA. The study thus demonstrated differential roles of Suc and auxin in the developmental responses of ontogenetically distinct root traits during Pi deprivation. In addition, lack of cross talk between local and systemic Pi sensing as revealed by the seedlings grown under either the Pi- Suc- condition or in the heterogeneous Pi environment highlighted the coexistence of Suc-independent and Suc-dependent regulatory mechanisms that constitute Pi starvation responses.
Figures





Similar articles
-
Increased Sucrose Accumulation Regulates Iron-Deficiency Responses by Promoting Auxin Signaling in Arabidopsis Plants.Plant Physiol. 2016 Feb;170(2):907-20. doi: 10.1104/pp.15.01598. Epub 2015 Dec 7. Plant Physiol. 2016. PMID: 26644507 Free PMC article.
-
A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis.Plant Physiol. 2005 Aug;138(4):2061-74. doi: 10.1104/pp.105.060061. Epub 2005 Jul 22. Plant Physiol. 2005. PMID: 16040660 Free PMC article.
-
Iron Availability Affects Phosphate Deficiency-Mediated Responses, and Evidence of Cross-Talk with Auxin and Zinc in Arabidopsis.Plant Cell Physiol. 2015 Jun;56(6):1107-23. doi: 10.1093/pcp/pcv035. Epub 2015 Mar 9. Plant Cell Physiol. 2015. PMID: 25759329
-
Signalling Overlaps between Nitrate and Auxin in Regulation of The Root System Architecture: Insights from the Arabidopsis thaliana.Int J Mol Sci. 2020 Apr 20;21(8):2880. doi: 10.3390/ijms21082880. Int J Mol Sci. 2020. PMID: 32326090 Free PMC article. Review.
-
Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response.J Exp Bot. 2013 Jun;64(9):2609-17. doi: 10.1093/jxb/ert155. J Exp Bot. 2013. PMID: 23709673 Review.
Cited by
-
Functional analysis of ZmPHR1 and ZmPHR2 under low-phosphate stress in maize.Mol Breed. 2024 Oct 1;44(10):69. doi: 10.1007/s11032-024-01508-2. eCollection 2024 Oct. Mol Breed. 2024. PMID: 39359407
-
Fast and abundant in vitro spontaneous haustorium formation in root hemiparasitic plant Pedicularis kansuensis Maxim. (Orobanchaceae).Plant Divers. 2018 Jul 24;40(5):226-231. doi: 10.1016/j.pld.2018.07.005. eCollection 2018 Oct. Plant Divers. 2018. PMID: 30740568 Free PMC article.
-
Light and Ethylene Coordinately Regulate the Phosphate Starvation Response through Transcriptional Regulation of PHOSPHATE STARVATION RESPONSE1.Plant Cell. 2017 Sep;29(9):2269-2284. doi: 10.1105/tpc.17.00268. Epub 2017 Aug 25. Plant Cell. 2017. PMID: 28842534 Free PMC article.
-
The promise of single-cell genomics in plants.Curr Opin Plant Biol. 2020 Apr;54:114-121. doi: 10.1016/j.pbi.2020.04.002. Epub 2020 May 5. Curr Opin Plant Biol. 2020. PMID: 32388018 Free PMC article. Review.
-
Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth.Planta. 2011 Jun;233(6):1129-43. doi: 10.1007/s00425-011-1368-1. Epub 2011 Feb 11. Planta. 2011. PMID: 21312041
References
-
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26 1053–1066
-
- Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8 115–118
-
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16 553–563 - PubMed
-
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

