Functional dissection of circuitry in a neural integrator
- PMID: 17369822
- PMCID: PMC2803116
- DOI: 10.1038/nn1877
Functional dissection of circuitry in a neural integrator
Erratum in
- Nat Neurosci. 2007 Jun;10(6):798
Abstract
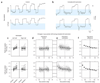
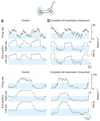
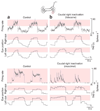
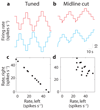
In neural integrators, transient inputs are accumulated into persistent firing rates that are a neural correlate of short-term memory. Integrators often contain two opposing cell populations that increase and decrease sustained firing as a stored parameter value rises. A leading hypothesis for the mechanism of persistence is positive feedback through mutual inhibition between these opposing populations. We tested predictions of this hypothesis in the goldfish oculomotor velocity-to-position integrator by measuring the eye position and firing rates of one population, while pharmacologically silencing the opposing one. In complementary experiments, we measured responses in a partially silenced single population. Contrary to predictions, induced drifts in neural firing were limited to half of the oculomotor range. We built network models with synaptic-input thresholds to demonstrate a new hypothesis suggested by these data: mutual inhibition between the populations does not provide positive feedback in support of integration, but rather coordinates persistent activity intrinsic to each population.
Figures




References
-
- Taube JS, Bassett JP. Persistent neural activity in head direction cells. Cereb. Cortex. 2003;13:1162–1172. - PubMed
-
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb. Cortex. 2003;13:1257–1269. - PubMed
-
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 2004;14:675–684. - PubMed
-
- Lopez-Barneo J, Darlot C, Berthoz A, Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J. Neurophysiol. 1982;47:329–352. - PubMed
-
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J. Neurophysiol. 1992;68:319–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

