Chitosan-DNA nanoparticles delivered by intrabiliary infusion enhance liver-targeted gene delivery
- PMID: 17369870
- PMCID: PMC1828073
- DOI: 10.2147/nano.2006.1.4.507
Chitosan-DNA nanoparticles delivered by intrabiliary infusion enhance liver-targeted gene delivery
Abstract
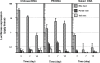
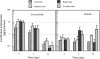
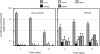
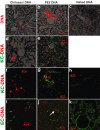
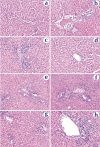
The goal of this study was to examine the efficacy of liver-targeted gene delivery by chitosan-DNA nanoparticles through retrograde intrabiliary infusion (RII). The transfection efficiency of chitosan-DNA nanoparticles, as compared with PEI-DNA nanoparticles or naked DNA, was evaluated in Wistar rats by infusion into the common bile duct, portal vein, or tail vein. Chitosan-DNA nanoparticles administrated through the portal vein or tail vein did not produce detectable luciferase expression. In contrast, rats that received chitosan-DNA nanoparticles showed more than 500 times higher luciferase expression in the liver 3 days after RII; and transgene expression levels decreased gradually over 14 days. Luciferase expression in the kidney, lung, spleen, and heart was negligible compared with that in the liver. RII of chitosan-DNA nanoparticles did not yield significant toxicity and damage to the liver and biliary tree as evidenced by liver function analysis and histopathological examination. Luciferase expression by RII of PEI-DNA nanoparticles was 17-fold lower than that of chitosan-DNA nanoparticles on day 3, but it increased slightly over time. These results suggest that RII is a promising routine to achieve liver-targeted gene delivery by non-viral nanoparticles; and both gene carrier characteristics and mode of administration significantly influence gene delivery efficiency.
Figures




References
-
- Asad SF, Singh S, Ahmad A, et al. Bilirubin-Cu(II) complex degrades DNA. Biochim Biophys Acta. 1999;1428:201–8. - PubMed
-
- Asad SF, Singh S, Ahmad A, et al. Bilirubin/biliverdin-Cu(II) induced DNA breakage; reaction mechanism and biological significance. Toxicol Lett. 2002;131:181–9. - PubMed
-
- Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52:145–50. - PubMed
-
- Bowman K, Sarkar R, Wang XL, et al. Evaluation of non-viral vectors for gene therapy of hemophilia A. Mol Ther. 2004;9:S314.
-
- Cheah PY, Bernstein H. Modification of DNA by bile acids: a possible factor in the etiology of colon cancer. Cancer Lett. 1990;49:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

