Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability
- PMID: 17370339
- PMCID: PMC6871473
- DOI: 10.1002/hbm.20364
Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability
Abstract
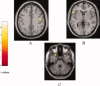
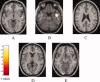
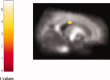
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the progressive and simultaneous degeneration of upper and lower motor neurons. The pathological process associated to ALS, albeit more pronounced in the motor/premotor cortices and along the corticospinal tracts (CST), does not spare extra-motor brain gray (GM) and white (WM) matter structures. However, it remains unclear whether such extra-motor cerebral abnormalities occur with mildly disabling disease, and how irreversible tissue loss and intrinsic tissue damage are interrelated. To this end, we used an optimized version of voxel-based morphometry (VBM) analysis to investigate the patterns of regional GM density changes and to quantify GM and WM diffusivity alterations of the entire brain from mildly disabled patients with ALS. A high-resolution T1-weighted 3D magnetization-prepared rapid acquisition gradient echo and a pulsed gradient spin-echo single shot echo-planar sequence of the brain were acquired from 25 mildly disabled patients with ALS and 18 matched healthy controls. An analysis of covariance was used to compare volumetry and diffusivity measurements between patients and controls. Compared with controls, ALS patients had significant clusters of locally reduced GM density (P < 0.001) in the right premotor cortex, left inferior frontal gyrus (IFG), and superior temporal gyrus (STG), bilaterally. In ALS patients contrasted to controls, we also found significant clusters of locally increased MD (P < 0.001) in the splenium of the corpus callosum and in the WM adjacent to the IFG, STG, and middle temporal gyrus (MTG) of the right hemisphere, and in the WM adjacent to the MTG and lingual gyrus in the left hemisphere. Compared with controls, ALS patients also had significant clusters of locally decreased FA values (P < 0.001) in the CST in the midbrain and corpus callosum, bilaterally. This study supports the notion that ALS is a multisystem disorder and suggests that extra-motor involvement may be an early feature of the disease.
(copyright) 2007 Wiley-Liss, Inc.
Figures
References
-
- Abe K,Fujimura H,Kobayashi Y,Fujita N,Yanagihara T ( 1997a): Degeneration of the corticospinal tracts in patients with amyotrophic lateral sclerosis. A premortem and postmortem magnetic resonance imaging study. J Neuroimaging 7: 208–212. - PubMed
-
- Abe K,Fujimura H,Toyooka K,Sakoda S,Yorifuji S,Yanagihara T ( 1997b): Cognitive function in amyotrophic lateral sclerosis. J Neurol Sci 148: 95–100. - PubMed
-
- Abe K,Takanashi M,Watanabe Y,Tanaka H,Fujita N,Hirabuki N,Yanagihara T ( 2001): Decrease in N‐acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology 43: 537–541. - PubMed
-
- Abe O,Yamada H,Masutani Y,Aoki S,Kunimatsu A,Yamasue H,Yamasue H,Fukuda R,Kasai K,Hayashi N,Masumoto T,Mori H,Soma T,Ohtomo K ( 2004): Amyotrophic lateral sclerosis: Diffusion tensor tractography and voxel‐based analysis. NMR Biomed 17: 411–416. - PubMed
-
- Abrahams S,Goldstein LH,Kew JJ,Brooks DJ,Lloyd CM,Frith CD,Leigh PN ( 1996): Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119: 2105–2120. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

