Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells
- PMID: 17371851
- PMCID: PMC1932862
- DOI: 10.1128/IAI.01935-06
Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells
Abstract
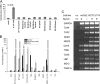
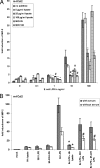
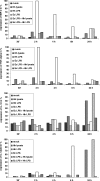
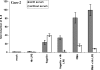
Enterohepatic Helicobacter species infect the intestinal tracts and biliary trees of various mammals, including mice and humans, and are associated with chronic inflammatory diseases of the intestine, gallstone formation, and malignant transformation. The recent analysis of the whole genome sequence of the mouse enterohepatic species Helicobacter hepaticus allowed us to perform a functional analysis of bacterial factors that may play a role in these diseases. We tested the hypothesis that H. hepaticus suppresses or evades innate immune responses of mouse intestinal epithelial cells, which allows this pathogen to induce or contribute to chronic inflammatory disease. We demonstrated in the present study that the innate immune responses of intestinal epithelial cells to lipopolysaccharide (LPS) via Toll-like receptor 4 (TLR4) and to flagellin-mediated activation via TLR5 are reduced by H. hepaticus infection through soluble bacterial factors. In particular, H. hepaticus lysate and the soluble component LPS antagonized TLR4- and TLR5-mediated immune responses of intestinal epithelial cells. H. hepaticus lysate and LPS inhibited development of endotoxin tolerance to Escherichia coli LPS. Suppression of innate immune responses by H. hepaticus LPS thus may affect intestinal responses to the resident microbial flora, epithelial homeostasis, and intestinal inflammatory conditions.
Figures



Similar articles
-
Hcp and VgrG1 are secreted components of the Helicobacter hepaticus type VI secretion system and VgrG1 increases the bacterial colitogenic potential.Cell Microbiol. 2013 Jun;15(6):992-1011. doi: 10.1111/cmi.12094. Epub 2013 Jan 17. Cell Microbiol. 2013. PMID: 23278999
-
Sex influence on chronic intestinal inflammation in Helicobacter hepaticus-infected A/JCr mice.Comp Med. 2004 Jun;54(3):301-8. Comp Med. 2004. PMID: 15253277
-
Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice.Cancer Res. 2006 Aug 1;66(15):7395-400. doi: 10.1158/0008-5472.CAN-06-0558. Cancer Res. 2006. PMID: 16885333
-
Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer.Mucosal Immunol. 2011 Jan;4(1):22-30. doi: 10.1038/mi.2010.61. Epub 2010 Oct 13. Mucosal Immunol. 2011. PMID: 20944559 Free PMC article. Review.
-
Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists.Biotechnol Adv. 2012 Jan-Feb;30(1):251-60. doi: 10.1016/j.biotechadv.2011.05.014. Epub 2011 Jun 6. Biotechnol Adv. 2012. PMID: 21664961 Review.
Cited by
-
Helicobacter hepaticus, a new pathogenic species of the Helicobacter genus: Similarities and differences with H. pylori.Iran J Microbiol. 2013 Sep;5(3):185-94. Iran J Microbiol. 2013. PMID: 24475322 Free PMC article. Review.
-
Differentiation of Gastric Helicobacter Species Using MALDI-TOF Mass Spectrometry.Pathogens. 2021 Mar 18;10(3):366. doi: 10.3390/pathogens10030366. Pathogens. 2021. PMID: 33803832 Free PMC article.
-
Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-κB-dependent pathway.PLoS One. 2011;6(12):e29007. doi: 10.1371/journal.pone.0029007. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216156 Free PMC article.
-
The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models.Nat Rev Microbiol. 2010 Aug;8(8):564-77. doi: 10.1038/nrmicro2403. Epub 2010 Jul 12. Nat Rev Microbiol. 2010. PMID: 20622892 Review.
-
TLR2-independent induction and regulation of chronic intestinal inflammation.Eur J Immunol. 2010 Feb;40(2):516-24. doi: 10.1002/eji.200939669. Eur J Immunol. 2010. PMID: 19950179 Free PMC article.
References
-
- Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174:4453-4460. - PubMed
-
- Andersen, L. P. 2001. New Helicobacter species in humans. Dig. Dis. 19:112-115. - PubMed
-
- Apostolov, E., W. A. Al Soud, I. Nilsson, I. Kornilovska, V. Usenko, V. Lyzogubov, Y. Gaydar, T. Wadstrom, and A. Ljungh. 2005. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand. J. Gastroenterol. 40:96-102. - PubMed
-
- Asai, Y., M. Hashimoto, and T. Ogawa. 2003. Treponemal glycoconjugate inhibits Toll-like receptor ligand-induced cell activation by blocking LPS-binding protein and CD14 functions. Eur. J. Immunol. 33:3196-3204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

