Moraxella catarrhalis strain O35E expresses two filamentous hemagglutinin-like proteins that mediate adherence to human epithelial cells
- PMID: 17371858
- PMCID: PMC1932885
- DOI: 10.1128/IAI.00079-07
Moraxella catarrhalis strain O35E expresses two filamentous hemagglutinin-like proteins that mediate adherence to human epithelial cells
Abstract
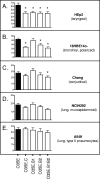
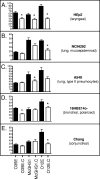
Two-partner secretion (TPS) systems are a family of proteins being rapidly identified and characterized in a growing number of gram-negative bacteria. TPS systems mediate the secretion of proteins, many involved in virulence traits such as hemolysis, adherence to epithelial cells, inhibition of bacterial growth, and immunomodulation of the host. A TPS system typically consists of a transporter located in the bacterial outer membrane (OM) which is responsible for the recognition and secretion of at least one large exoprotein. Two of the better-characterized TPS systems specify the Bordetella pertussis FHA and Haemophilus influenzae HMW1/HMW2 proteins. We identified three gene products of Moraxella catarrhalis strain O35E that resemble TPS proteins and designated them MhaC (transporter), MhaB1 (exoprotein), and MhaB2 (exoprotein). Western blot analysis using anti-MhaC, or antibodies reacting to both MhaB1 and MhaB2 (MhaB-reactive), revealed that these antigens are expressed in the OM of 63% of isolates tested. Mutations in the mhaC gene specifying the putative transporter of the M. catarrhalis wild-type strains O35E, O12E, and McGHS1 resulted in the absence of MhaB1/MhaB2 in the OM of mutants. These results are therefore consistent with the Mha proteins functioning as a TPS system. Furthermore, we discovered that these mhaC mutants exhibit markedly decreased binding to human epithelial cells relevant to pathogenesis by M. catarrhalis (Chang, HEp2, A549, and/or 16HBE14o(-)). Expression of O12E MhaC and MhaB1 in a nonadherent strain of Escherichia coli was found to increase the adherence of recombinant bacteria to HEp2 monolayers by sevenfold, thereby demonstrating that this M. catarrhalis TPS system directly mediates binding to human epithelial cells. The construction of isogenic mutants in the mhaB1 and mhaB2 genes of strain O35E also suggests that the MhaB proteins play distinct roles in M. catarrhalis adherence.
Figures




References
-
- Atkinson, W., J. Hamborsky, L. McIntyre, and S. Wolfe (ed.). 2007. Epidemiology and prevention of vaccine-preventable diseases, 10th ed., p. 81-100. Public Health Foundation, Washington, DC. http://www.cdc.gov/nip/publications/pink/pert.pdf.
-
- Barenkamp, S. J., and J. W. St Geme III. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215-1223. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

