Candida albicans Iff11, a secreted protein required for cell wall structure and virulence
- PMID: 17371861
- PMCID: PMC1932888
- DOI: 10.1128/IAI.00102-07
Candida albicans Iff11, a secreted protein required for cell wall structure and virulence
Abstract
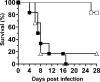
The Candida albicans cell wall is the immediate point of contact with the host and is implicated in the host-fungal interaction and virulence. To date, a number of cell wall proteins have been identified and associated with virulence. Analysis of the C. albicans genome has identified the IFF gene family as encoding the largest family of cell wall-related proteins. This family is also conserved in a range of other Candida species. Iff11 differs from other family members in lacking a GPI anchor, and we have demonstrated it to be O glycosylated and secreted in C. albicans. A null mutant lacking IFF11 was hypersensitive to cell wall-damaging agents, suggesting a role in cell wall organization. In a murine model of systemic infection the null mutant was highly attenuated in virulence, and survival-standardized infections suggest it is required to establish an infection. This work provides the first evidence of the importance of this gene family in the host-fungal interaction and virulence.
Figures
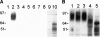
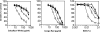
References
-
- Bates, S., H. B. Hughes, C. A. Munro, W. P. Thomas, D. M. MacCallum, G. Bertram, A. Atrih, M. A. Ferguson, A. J. Brown, F. C. Odds, and N. A. Gow. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90-98. - PubMed
-
- Bates, S., D. M. MacCallum, G. Bertram, C. A. Munro, H. B. Hughes, E. T. Buurman, A. J. Brown, F. C. Odds, and N. A. Gow. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280:23408-23415. - PubMed
-
- Calderone, R. A. (ed.). 2002. Candida and candidiasis. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

