MyD88-dependent signals are essential for the host immune response in experimental brain abscess
- PMID: 17372011
- PMCID: PMC2094730
- DOI: 10.4049/jimmunol.178.7.4528
MyD88-dependent signals are essential for the host immune response in experimental brain abscess
Abstract
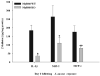
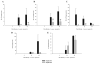
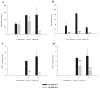
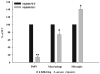
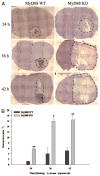
Brain abscesses form in response to a parenchymal infection by pyogenic bacteria, with Staphylococcus aureus representing a common etiologic agent of human disease. Numerous receptors that participate in immune responses to bacteria, including the majority of TLRs, the IL-1R, and the IL-18R, use a common adaptor molecule, MyD88, for transducing activation signals leading to proinflammatory mediator expression and immune effector functions. To delineate the importance of MyD88-dependent signals in brain abscesses, we compared disease pathogenesis using MyD88 knockout (KO) and wild-type (WT) mice. Mortality rates were significantly higher in MyD88 KO mice, which correlated with a significant reduction in the expression of several proinflammatory mediators, including but not limited to IL-1beta, TNF-alpha, and MIP-2/CXCL2. These changes were associated with a significant reduction in neutrophil and macrophage recruitment into brain abscesses of MyD88 KO animals. In addition, microglia, macrophages, and neutrophils isolated from the brain abscesses of MyD88 KO mice produced significantly less TNF-alpha, IL-6, MIP-1alpha/CCL3, and IFN-gamma-induced protein 10/CXCL10 compared with WT cells. The lack of MyD88-dependent signals had a dramatic effect on the extent of tissue injury, with significantly larger brain abscesses typified by exaggerated edema and necrosis in MyD88 KO animals. Interestingly, despite these striking changes in MyD88 KO mice, bacterial burdens did not significantly differ between the two strains at the early time points examined. Collectively, these findings indicate that MyD88 plays an essential role in establishing a protective CNS host response during the early stages of brain abscess development, whereas MyD88-independent pathway(s) are responsible for pathogen containment.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
References
-
- Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005;95:254–262. - PubMed
-
- Lo WD, Wolny A, Boesel C. Blood-brain barrier permeability in staphylococcal cerebritis and early brain abscess. J Neurosurg. 1994;80:897–905. - PubMed
-
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

