Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency
- PMID: 17372190
- PMCID: PMC1838514
- DOI: 10.1073/pnas.0701331104
Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency
Abstract
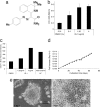
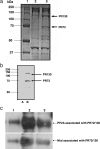
Embryonic stem cells (ESCs) represent an important research tool and a potential resource for regenerative medicine. Generally, ESCs are cocultured with a supportive feeder cell layer of murine embryonic fibroblasts, which maintain the ESCs' capacity for self-renewal and block spontaneous differentiation. These cumbersome conditions, as well as the risk of xenobiotic contamination of human ESCs grown on murine embryonic fibroblasts, make it a priority to develop chemically defined methods that can be safely used for the expansion of ESCs. Using a high-throughput, cell-based assay, we identified the small molecule IQ-1 that allows for the Wnt/beta-catenin-driven long-term expansion of mouse ESCs and prevents spontaneous differentiation. We demonstrate that IQ-1, by targeting the PR72/130 subunit of the serine/threonine phosphatase PP2A, prevents beta-catenin from switching coactivator usage from CBP to p300. The increase in beta-catenin/CBP-mediated transcription at the expense of beta-catenin/p300-mediated transcription is critical for the maintenance of murine stem cell pluripotency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

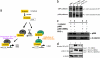

References
-
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Stem Cells. 2005;23:1489–1501. - PubMed
-
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Nature. 2003;423:448–452. - PubMed
-
- Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S. Mol Cell Neurosci. 2003;24:696–708. - PubMed
-
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou A. Nat Med. 2004;10:55–63. - PubMed
-
- Chenn A, Walsh CA. Science. 2002;297:365–369. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

