Perturbed ATPase activity and not "close confinement" of substrate in the cis cavity affects rates of folding by tail-multiplied GroEL
- PMID: 17372195
- PMCID: PMC1828711
- DOI: 10.1073/pnas.0700820104
Perturbed ATPase activity and not "close confinement" of substrate in the cis cavity affects rates of folding by tail-multiplied GroEL
Abstract
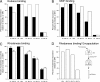
Folding of substrate proteins inside the sequestered and hydrophilic GroEL-GroES cis cavity favors production of the native state. Recent studies of GroEL molecules containing volume-occupying multiplications of the flexible C-terminal tail segments have been interpreted to indicate that close confinement of substrate proteins in the cavity optimizes the rate of folding: the rate of folding of a larger protein, Rubisco (51 kDa), was compromised by multiplication, whereas that of a smaller protein, rhodanese (33 kDa), was increased by tail duplication. Here, we report that this latter effect does not extend to the subunit of malate dehydrogenase (MDH), also 33 kDa. In addition, single-ring versions of tail-duplicated and triplicated molecules, comprising stable cis complexes, did not produce any acceleration of folding of rhodanese or MDH, nor did they show significant retardation of the folding of Rubisco. Tail quadruplication produced major reduction in recovery of native protein with both systems, the result of strongly reduced binding of all three substrates. When steady-state ATPase of the tail-multiplied double-ring GroELs was examined, it scaled directly with the number of tail segments, with more than double the normal ATPase rate upon tail triplication. As previously observed, disturbance of ATPase activity of the cycling double-ring system, and thus of "dwell time" for the folding protein in the cis cavity, produces effects on folding rates. We conclude that, within the limits of the approximately 10% decrease of cavity volume produced by tail triplication, there does not appear to be an effect of "close confinement" on folding in the cis cavity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

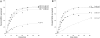

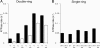
Similar articles
-
Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL.Nature. 1997 Aug 21;388(6644):792-8. doi: 10.1038/42047. Nature. 1997. PMID: 9285593
-
Multivalent binding of nonnative substrate proteins by the chaperonin GroEL.Cell. 2000 Mar 3;100(5):561-73. doi: 10.1016/s0092-8674(00)80692-3. Cell. 2000. PMID: 10721993
-
Functional characterization of an archaeal GroEL/GroES chaperonin system: significance of substrate encapsulation.J Biol Chem. 2004 Jan 9;279(2):1090-9. doi: 10.1074/jbc.M310914200. Epub 2003 Oct 23. J Biol Chem. 2004. PMID: 14576149
-
Reaction Cycle of Chaperonin GroEL via Symmetric "Football" Intermediate.J Mol Biol. 2015 Sep 11;427(18):2912-8. doi: 10.1016/j.jmb.2015.04.007. Epub 2015 Apr 18. J Mol Biol. 2015. PMID: 25900372 Review.
-
Structure and function in GroEL-mediated protein folding.Annu Rev Biochem. 1998;67:581-608. doi: 10.1146/annurev.biochem.67.1.581. Annu Rev Biochem. 1998. PMID: 9759498 Review.
Cited by
-
Indole-3-glycerol-phosphate synthase is recognized by a cold-inducible group II chaperonin in Thermococcus kodakarensis.Appl Environ Microbiol. 2012 Jun;78(11):3806-15. doi: 10.1128/AEM.07996-11. Epub 2012 Mar 23. Appl Environ Microbiol. 2012. PMID: 22447592 Free PMC article.
-
Physicochemical and thermodynamic properties of purified rhodanese from A. welwitschiae LOT1 and the cyanide detoxification potential of the enzyme.World J Microbiol Biotechnol. 2024 Oct 19;40(11):355. doi: 10.1007/s11274-024-04164-y. World J Microbiol Biotechnol. 2024. PMID: 39424675
-
Single-molecule spectroscopy of protein folding in a chaperonin cage.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11793-8. doi: 10.1073/pnas.1002356107. Epub 2010 Jun 14. Proc Natl Acad Sci U S A. 2010. PMID: 20547872 Free PMC article.
-
Essential role of the chaperonin folding compartment in vivo.EMBO J. 2008 May 21;27(10):1458-68. doi: 10.1038/emboj.2008.77. Epub 2008 Apr 17. EMBO J. 2008. PMID: 18418386 Free PMC article.
-
Role of nonspecific interactions in molecular chaperones through model-based bioinformatics.Biophys J. 2012 Dec 19;103(12):2484-91. doi: 10.1016/j.bpj.2012.10.040. Epub 2012 Dec 18. Biophys J. 2012. PMID: 23260050 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

