Synthesis of the next-generation therapeutic antibodies that combine cell targeting and antibody-catalyzed prodrug activation
- PMID: 17372220
- PMCID: PMC1838444
- DOI: 10.1073/pnas.0700223104
Synthesis of the next-generation therapeutic antibodies that combine cell targeting and antibody-catalyzed prodrug activation
Abstract
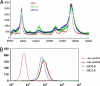
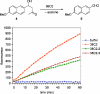
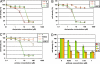
An obstacle in the utilization of catalytic Abs for selective prodrug activation in cancer therapy has been systemic tumor targeting. Here we report the generation of catalytic Abs that effectively target tumor cells with undiminished prodrug activation capability. Ab conjugates were prepared by covalent conjugation of an integrin alpha(v)beta(3)-targeting antagonist to catalytic Ab 38C2 through either sulfide groups of cysteine residues generated by reduction of the disulfide bridges in the hinge region or surface lysine residues not involved in the catalytic activity. Using flow cytometry, the Ab conjugates were shown to bind efficiently to integrin alpha(v)beta(3)-expressing human breast cancer cells. The Ab conjugates also retained the retro-aldol activity of their parental catalytic Ab 38C2, as measured by methodol and doxorubicin (dox) prodrug activation. Complementing these Ab conjugates, an evolved set of dox prodrugs was designed and synthesized. Dox prodrugs that showed higher stability and lower toxicity were evaluated both in the presence and absence of the integrin alpha(v)beta(3)-targeting 38C2 conjugates for cell-killing efficacy by using human breast cancer cells. Our study reveals that cell targeting and prodrug activation capabilities can be efficiently combined for selective chemotherapy with novel dox prodrugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

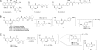


References
-
- Tanaka F, Barbas CF., III . In: Catalytic Antibodies. Keinan E, editor. Weinheim, Germany: Wiley-VCH; 2005. p. 304.
-
- Zhong G, Shabat D, List B, Anderson J, Sinha SC, Lerner RA, Barbas CF., III Angew Chem Int Ed. 1998;37:2481–2484. - PubMed
-
- Sinha SC, Sun J, Miller G, Barbas CF, III, Lerner RA. Org Lett. 1999;1:1623–1626. - PubMed
-
- Wagner J, Lerner RA, Barbas CF., III Science. 1995;270:1797–1800. - PubMed
-
- Zhong G, Lerner RA, Barbas CF., III Angew Chem Int Ed. 1999;38:3738–3741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

