Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood
- PMID: 17372227
- PMCID: PMC1838510
- DOI: 10.1073/pnas.0609809104
Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood
Abstract
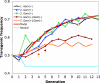
The introduction of genes that impair Plasmodium development into mosquito populations is a strategy being considered for malaria control. The effect of the transgene on mosquito fitness is a crucial parameter influencing the success of this approach. We have previously shown that anopheline mosquitoes expressing the SM1 peptide in the midgut lumen are impaired for transmission of Plasmodium berghei. Moreover, the transgenic mosquitoes had no noticeable fitness load compared with nontransgenic mosquitoes when fed on noninfected mice. Here we show that when fed on mice infected with P. berghei, these transgenic mosquitoes are more fit (higher fecundity and lower mortality) than sibling nontransgenic mosquitoes. In cage experiments, transgenic mosquitoes gradually replaced nontransgenics when mosquitoes were maintained on mice infected with gametocyte-producing parasites (strain ANKA 2.34) but not when maintained on mice infected with gametocyte-deficient parasites (strain ANKA 2.33). These findings suggest that when feeding on Plasmodium-infected blood, transgenic malaria-resistant mosquitoes have a selective advantage over nontransgenic mosquitoes. This fitness advantage has important implications for devising malaria control strategies by means of genetic modification of mosquitoes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Nature. 2002;417:452–455. - PubMed
-
- James AA. Trends Parasitol. 2005;21:64–67. - PubMed
-
- Christophides GK. Cell Microbiol. 2005;7:325–333. - PubMed
-
- Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. J Biol Chem. 2002;277:40839–40843. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

