A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis
- PMID: 17372230
- PMCID: PMC1828708
- DOI: 10.1073/pnas.0700809104
A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis
Abstract
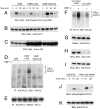
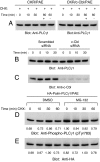
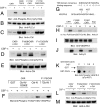
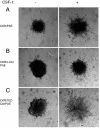
Activation of phospholipase Cgamma1 (PLCgamma1) by vascular endothelial growth factor receptor-2 (VEGFR-2) in endothelial cells in part is responsible for angiogenesis in vivo. The cellular mechanisms exerting negative control over PLCgamma1 activation, however, remain unaddressed. Here by using in vitro and in vivo binding assays, we show that the Casitas B-lineage lymphoma (c-Cbl) E3 ubiquitin ligase constitutively associates with PLCgamma1 via its C-terminal domain and conditionally interacts with VEGFR-2 via the N-terminal/TKB domain. Site-directed mutagenesis of VEGFR-2 showed that full activation of c-Cbl requires its direct association with phospho-tyrosines 1052 and 1057 of VEGFR-2 via its TKB domain and indirect association with phospho-tyrosine 1173 of VEGFR-2 via PLCgamma1. The tertiary complex formation between VEGFR-2, PLCgamma1 and c-Cbl selectively promotes ubiquitylation and suppression of tyrosine phosphorylation of PLCgamma1 by a proteolysis-independent mechanism. Further analysis showed that association of c-Cbl with VEGFR-2 does not impact ubiquitylation, down-regulation, or tyrosine phosphorylation of VEGFR-2. Silencing of c-Cbl by siRNA revealed that endogenous c-Cbl plays an inhibitory role in angiogenesis. Our data demonstrate that corecruitment of c-Cbl and PLCgamma1 to VEGFR-2 serves as a mechanism to fine-tune the angiogenic signal relay of VEGFR-2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCγ1.Oncogene. 2011 May 12;30(19):2198-206. doi: 10.1038/onc.2010.597. Epub 2011 Jan 17. Oncogene. 2011. PMID: 21242968 Free PMC article.
-
Role of c-Cbl-dependent regulation of phospholipase Cgamma1 activation in experimental choroidal neovascularization.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6803-9. doi: 10.1167/iovs.10-5255. Epub 2010 Jun 30. Invest Ophthalmol Vis Sci. 2010. PMID: 20592236 Free PMC article.
-
A role for protein ubiquitination in VEGFR-2 signalling and angiogenesis.Biochem Soc Trans. 2009 Dec;37(Pt 6):1189-92. doi: 10.1042/BST0371189. Biochem Soc Trans. 2009. PMID: 19909244
-
c-Cbl: An Important Regulator and a Target in Angiogenesis and Tumorigenesis.Cells. 2019 May 23;8(5):498. doi: 10.3390/cells8050498. Cells. 2019. PMID: 31126146 Free PMC article. Review.
-
Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials.Exp Eye Res. 2006 Nov;83(5):1005-16. doi: 10.1016/j.exer.2006.03.019. Epub 2006 May 19. Exp Eye Res. 2006. PMID: 16713597 Free PMC article. Review.
Cited by
-
Clear Cell Renal Carcinoma: MicroRNAs With Efficacy in Preclinical In Vivo Models.Cancer Genomics Proteomics. 2021 May-Jun;18(3 Suppl):349-368. doi: 10.21873/cgp.20265. Epub 2021 May 15. Cancer Genomics Proteomics. 2021. PMID: 33994361 Free PMC article. Review.
-
c-Casitas b-Lineage Lymphoma Downregulation Improves the Ability of Long-term Cultured Mesenchymal Stem Cells for Promoting Angiogenesis and Diabetic Wound Healing.Cell Transplant. 2021 Jan-Dec;30:963689721989605. doi: 10.1177/0963689721989605. Cell Transplant. 2021. PMID: 33588607 Free PMC article.
-
c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCγ1.Oncogene. 2011 May 12;30(19):2198-206. doi: 10.1038/onc.2010.597. Epub 2011 Jan 17. Oncogene. 2011. PMID: 21242968 Free PMC article.
-
Eimeria acervulina Microneme Protein 3 Inhibits Apoptosis of the Chicken Duodenal Epithelial Cell by Targeting the Casitas B-Lineage Lymphoma Protein.Front Vet Sci. 2021 May 18;8:636809. doi: 10.3389/fvets.2021.636809. eCollection 2021. Front Vet Sci. 2021. PMID: 34141730 Free PMC article.
-
Role of E3 ubiquitin ligases in lung cancer.World J Clin Oncol. 2013 Aug 10;4(3):58-69. doi: 10.5306/wjco.v4.i3.58. World J Clin Oncol. 2013. PMID: 23936758 Free PMC article.
References
-
- Jain R. Nat Med. 2003;9:685–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

