GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo
- PMID: 17373658
- PMCID: PMC1971132
- DOI: 10.1002/bies.20558
GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo
Abstract
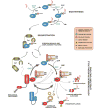
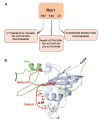
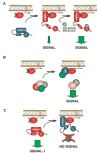
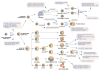
Rho/Rac proteins constitute a subgroup of the Ras superfamily of GTP hydrolases. Although originally implicated in the control of cytoskeletal events, it is currently known that these GTPases coordinate diverse cellular functions, including cell polarity, vesicular trafficking, the cell cycle and transcriptomal dynamics. In this review, we will provide an overview on the recent advances in this field regarding the mechanism of regulation and signaling, and the roles in vivo of this important GTPase family.
(c) 2007 Wiley Periodicals, Inc.
Figures

References
-
- Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31–40. - PubMed
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. - PubMed
-
- Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. - PubMed
-
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. - PubMed
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

