Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease
- PMID: 17373741
- PMCID: PMC4146869
- DOI: 10.3748/wjg.v13.i7.1067
Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease
Abstract
Aim: To evaluate the safety and feasibility of bone marrow cell (BMC) transplantation in patients with chronic liver disease on the waiting list for liver transplantation.
Methods: Ten patients (eight males) with chronic liver disease were enrolled to receive infusion of autologous bone marrow-derived cells. Seven patients were classified as Child-Pugh B and three as Child-Pugh C. Baseline assessment included complete clinical and laboratory evaluation and abdominal MRI. Approximately 50 mL of bone marrow aspirate was prepared by centrifugation in a ficoll-hypaque gradient. At least of 100 millions of mononuclear-enriched BMCs were infused into the hepatic artery using the routine technique for arterial chemoembolization for liver tumors. Patients were followed up for adverse events up to 4 mo.
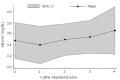
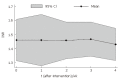
Results: The median age of the patients was 52 years (range 24-70 years). All patients were discharged 48 h after BMC infusion. Two patients complained of mild pain at the bone marrow needle puncture site. No other complications or specific side effects related to the procedure were observed. Bilirubin levels were lower at 1 (2.19 +/- 0.9) and 4 mo (2.10 +/- 1.0) after cell transplantation that baseline levels (2.78 +/- 1.2). Albumin levels 4 mo after BMC infusion (3.73 +/- 0.5) were higher than baseline levels (3.47 +/- 0.5). International normalized ratio (INR) decreased from 1.48 (SD = 0.23) to 1.43 (SD = 0.23) one month after cell transplantation.
Conclusion: BMC infusion into hepatic artery of patients with advanced chronic liver disease is safe and feasible. In addition, a decrease in mean serum bilirubin and INR levels and an increase in albumin levels are observed. Our data warrant further studies in order to evaluate the effect of BMC transplantation in patients with advanced chronic liver disease.
Figures




References
-
- Allen KJ, Buck NE, Williamson R. Stem cells for the treatment of liver disease. Transpl Immunol. 2005;15:99–112. - PubMed
-
- Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005;37:285–286. - PubMed
-
- Haque S, Haruna Y, Saito K, Nalesnik MA, Atillasoy E, Thung SN, Gerber MA. Identification of bipotential progenitor cells in human liver regeneration. Lab Invest. 1996;75:699–705. - PubMed
-
- Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. - PubMed
-
- Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology. 1997;25:329–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

