Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery
- PMID: 17373820
- PMCID: PMC2533281
- DOI: 10.1021/mp060122j
Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery
Abstract
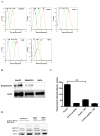
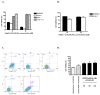
Major obstacles in the development of new therapeutic anticancer drugs are the low bioavailability of hydrophilic substances and the nonspecific toxicity toward healthy tissues. As such, cell-targeting oligopeptides have emerged as attractive drug delivery vehicles for a variety of different types of cargo. The recently identified peptide Pep42 binds to the glucose-regulated protein 78 (GRP78), which is overexpressed on the cell surface of human cancer cells and internalizes into these cells. Herein, we demonstrate how Pep42 can be utilized as a carrier for different types of cytotoxic drugs to specifically target human cancer cell lines in vitro in a strictly GRP78-dependent manner. Furthermore, the mechanism of internalization of Pep42 was elucidated and found to involve clathrin-mediated endocytosis. Pep42 subsequently colocalizes within the lysosomal compartment. Importantly, we also provide evidence that Pep42-conjugated quantum dots have the ability to selectively enrich in tumor tissue in a xenograft mouse model. Our results suggest that the highly specific GRP78-Pep42 interaction can be utilized for the generation of Pep42-drug conjugates as a powerful anticancer drug delivery system that could substantially enhance the efficacy of chemotherapy by increasing the drug-tumor specificity, thus minimizing the adverse side effects associated with conventional cancer therapeutics.
Figures






Similar articles
-
Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand.Biochemistry. 2006 Aug 8;45(31):9434-44. doi: 10.1021/bi060264j. Biochemistry. 2006. PMID: 16878978
-
A cell-penetrating peptidic GRP78 ligand for tumor cell-specific prodrug therapy.Bioorg Med Chem Lett. 2008 Mar 1;18(5):1632-6. doi: 10.1016/j.bmcl.2008.01.060. Epub 2008 Jan 19. Bioorg Med Chem Lett. 2008. PMID: 18243696 Free PMC article.
-
Cell-Penetrating Peptidic GRP78 Ligand-Conjugated Iron Oxide Magnetic Nanoparticles for Tumor-Targeted Doxorubicin Delivery and Imaging.ACS Appl Bio Mater. 2023 Mar 20;6(3):1019-1031. doi: 10.1021/acsabm.2c00897. Epub 2023 Mar 2. ACS Appl Bio Mater. 2023. PMID: 36862384
-
GRP78 in lung cancer.J Transl Med. 2021 Mar 21;19(1):118. doi: 10.1186/s12967-021-02786-6. J Transl Med. 2021. PMID: 33743739 Free PMC article. Review.
-
Stress induction of GRP78/BiP and its role in cancer.Curr Mol Med. 2006 Feb;6(1):45-54. doi: 10.2174/156652406775574523. Curr Mol Med. 2006. PMID: 16472112 Review.
Cited by
-
GRP78 targeting: Hitting two birds with a stone.Life Sci. 2020 Nov 1;260:118317. doi: 10.1016/j.lfs.2020.118317. Epub 2020 Aug 22. Life Sci. 2020. PMID: 32841659 Free PMC article. Review.
-
Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP.PLoS One. 2009 Aug 31;4(8):e6868. doi: 10.1371/journal.pone.0006868. PLoS One. 2009. PMID: 19718440 Free PMC article.
-
Tunicamycin-induced ER stress in breast cancer cells neither expresses GRP78 on the surface nor secretes it into the media.Glycobiology. 2018 Feb 1;28(2):61-68. doi: 10.1093/glycob/cwx098. Glycobiology. 2018. PMID: 29206917 Free PMC article.
-
Probing the ATP Site of GRP78 with Nucleotide Triphosphate Analogs.PLoS One. 2016 May 4;11(5):e0154862. doi: 10.1371/journal.pone.0154862. eCollection 2016. PLoS One. 2016. PMID: 27144892 Free PMC article.
-
Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells.J Biol Chem. 2008 Apr 25;283(17):11752-62. doi: 10.1074/jbc.M708849200. Epub 2008 Feb 21. J Biol Chem. 2008. PMID: 18292083 Free PMC article.
References
-
- Langerak AD. Chemotherapy Regimens and Cancer Care. Landes Bioscience; Georgetown, TX: 2001. p. 209.
-
- Zhou X, Chang YC, Oyama T, McGuire MJ, Brown KC. J Am Chem Soc. 2004;126(48):15656–7. - PubMed
-
- Torchilin VP, Levchenko TS. Curr Protein Pept Sci. 2003;4(2):133–40. - PubMed
-
- Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Nat Biotechnol. 2000;18(4):410–4. - PubMed
-
- El-Andaloussi S, Holm T, Langel U. Curr Pharm Des. 2005;11(28):3597–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

