Fly photoreceptors demonstrate energy-information trade-offs in neural coding
- PMID: 17373859
- PMCID: PMC1828148
- DOI: 10.1371/journal.pbio.0050116
Fly photoreceptors demonstrate energy-information trade-offs in neural coding
Abstract
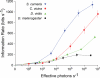
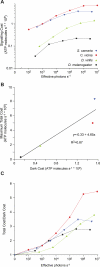
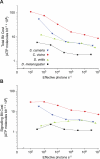
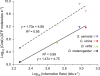
Trade-offs between energy consumption and neuronal performance must shape the design and evolution of nervous systems, but we lack empirical data showing how neuronal energy costs vary according to performance. Using intracellular recordings from the intact retinas of four flies, Drosophila melanogaster, D. virilis, Calliphora vicina, and Sarcophaga carnaria, we measured the rates at which homologous R1-6 photoreceptors of these species transmit information from the same stimuli and estimated the energy they consumed. In all species, both information rate and energy consumption increase with light intensity. Energy consumption rises from a baseline, the energy required to maintain the dark resting potential. This substantial fixed cost, approximately 20% of a photoreceptor's maximum consumption, causes the unit cost of information (ATP molecules hydrolysed per bit) to fall as information rate increases. The highest information rates, achieved at bright daylight levels, differed according to species, from approximately 200 bits s(-1) in D. melanogaster to approximately 1,000 bits s(-1) in S. carnaria. Comparing species, the fixed cost, the total cost of signalling, and the unit cost (cost per bit) all increase with a photoreceptor's highest information rate to make information more expensive in higher performance cells. This law of diminishing returns promotes the evolution of economical structures by severely penalising overcapacity. Similar relationships could influence the function and design of many neurons because they are subject to similar biophysical constraints on information throughput.
Conflict of interest statement
Figures



References
-
- Krebs JR, Davies NB. An introduction to behavioural ecology. Oxford: Blackwell Scientific; 1993. 420
-
- Alexander RM. Optima for animals. Princeton: Princeton University Press; 1996. 169
-
- Andersson M. Female choice selects for extreme tail length in a widowbird. Nature. 1982;299:818–820.
-
- Balmford A, Thomas ALR, Jones IL. Aerodynamics and the evolution of long tails in birds. Nature. 1993;361:628–631.
-
- Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain Mind. 2000;1:7–23.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

