Improvement of a low pH antigen-antibody dissociation procedure for ELISA measurement of circulating anti-Abeta antibodies
- PMID: 17374155
- PMCID: PMC1845169
- DOI: 10.1186/1471-2202-8-22
Improvement of a low pH antigen-antibody dissociation procedure for ELISA measurement of circulating anti-Abeta antibodies
Abstract
Background: Prior work from our group found that acid dissociation (pH 2.5 incubation) of serum from APP transgenic mice vaccinated against Abeta increased the apparent anti-Abeta titers, suggesting antibody masking by antigen in the ELISA assay. Subsequently, we found that pH 2.5 incubation of serum from unvaccinated non-transgenic mice showed antibody binding to Abeta1-42, but no increase when other proteins, including shorter Abeta peptides, coated the ELISA plate. To investigate further the effects of low pH incubation on apparent anti-Abeta1-42 signals, we examined normal sera from nonTg unvaccinated mice, nonTg mice vaccinated with Abeta peptide (to produce authentic anti-Abeta antibodies) or a monoclonal antibody against Abeta (6E10) using competitive-inhibition ELISA and Abeta epitope mapping assays. In addition, we examined use of a less stringent low pH procedure at pH 3.5, to ascertain if it had the same effects as the pH 2.5 procedure.
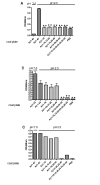
Results: We believe there are three distinct effects of pH 2.5 incubation.; A) an artifactual increase in binding to full length Abeta by mouse immunoglobulin which has low affinity for Abeta, B) an inactivation of anti-Abeta antibodies that is time dependent and C) unmasking of high affinity anti-Abeta antibodies when high levels of circulating Abeta is present in APP transgenic mice. All three reactions can interact to produce the final ELISA signal. Incubation of sera from unvaccinated nonTg mice at pH 2.5 enhanced ELISA signals by process A. Conversely, pH 2.5 incubation of sera from vaccinated nonTg mice with caused a time dependent reduction of antibody signal by process B (overcoming the increase caused by A). The artifactual anti-Abeta ELISA signal enhanced by pH 2.5 incubation of normal mouse sera could not be effectively competed by low to moderate concentrations of Abeta, nor bind to shorter Abeta peptides in a manner similar to authentic anti-Abeta antibodies. Incubation of mouse sera at pH 3.5 caused neither an apparent increase in anti-Abeta ELISA signal, nor an inactivation of the ELISA signals resulting from either vaccination or monoclonal antibodies. However, incubation at pH 3.5 was able to completely reverse the reduction in ELISA signal caused by Abeta complexing with antibodies in sera from vaccinated mice or monoclonal anti-Abeta antibodies.
Conclusion: Incubation at pH 3.5 is sufficient to dissociate Abeta bound to anti-Abeta antibodies without producing artifactual increases in the signal, or inactivating authentic antibody binding. Thus, use of pH 3.5 is a considerable improvement over pH 2.5 incubation for unmasking anti-Abeta antibodies in ELISA assays to measure antibodies in APP transgenic mouse sera.
Figures
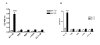
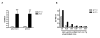
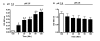


References
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. - DOI - PubMed
-
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. - DOI - PubMed
-
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. - DOI - PubMed
-
- Wilcock DM, Gordon MN, Ugen KE, Gottschall PE, DiCarlo G, Dickey C, Boyett KW, Jantzen PT, Connor KE, Melachrino J, Hardy J, Morgan D. Number of Abeta inoculations in APP+PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA Cell Biol. 2001;20:731–736. doi: 10.1089/10445490152717596. - DOI - PubMed
-
- Li QY, Cao CH, Chackerian B, Schiller J, Gordon M, Ugen KE, Morgan D. Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice. BMC Neurosci. 2004;5:21. doi: 10.1186/1471-2202-5-21. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

