Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy
- PMID: 17375050
- PMCID: PMC2360149
- DOI: 10.1038/sj.bjc.6603685
Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy
Abstract
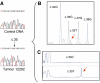
The predictive value of KRAS mutation in metastatic colorectal cancer (MCRC) patients treated with cetuximab plus chemotherapy has recently been suggested. In our study, 59 patients with a chemotherapy-refractory MCRC treated with cetuximab plus chemotherapy were included and clinical response was evaluated according to response evaluation criteria in solid tumours (RECIST). Tumours were screened for KRAS mutations using first direct sequencing, then two sensitive methods based on SNaPshot and PCR-ligase chain reaction (LCR) assays. Clinical response was evaluated according to gene mutations using the Fisher exact test. Times to progression (TTP) were calculated using the Kaplan-Meier method and compared with log-rank test. A KRAS mutation was detected in 22 out of 59 tumours and, in six cases, was missed by sequencing analysis but detected using the SNaPshot and PCR-LCR assays. Remarkably, no KRAS mutation was found in the 12 patients with clinical response. KRAS mutation was associated with disease progression (P=0.0005) and TTP was significantly decreased in mutated KRAS patients (3 vs 5.5 months, P=0.015). Our study confirms that KRAS mutation is highly predictive of a non-response to cetuximab plus chemotherapy in MCRC and highlights the need to use sensitive molecular methods, such as SNaPshot or PCR-LCR assays, to ensure an efficient mutation detection.
Figures
Comment in
-
Clinical interest of KRAS mutation detection in blood for anti-EGFR therapies in metastatic colorectal cancer.Br J Cancer. 2008 Aug 5;99(3):551-2. doi: 10.1038/sj.bjc.6604451. Epub 2008 Jul 1. Br J Cancer. 2008. PMID: 18594536 Free PMC article. No abstract available.
References
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345 - PubMed
-
- Douillard JY, Sobrero A, Carnaghi C, Comella P, Diaz-Rubio E, Santoro A, Van Cutsem E (2003) Metastatic colorectal cancer: integrating irinotecan into combination and sequential chemotherapy. Ann Oncol 14(Suppl 2): ii7–ii12 - PubMed
-
- Hurwitz H, Fehrenbacher I, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 - PubMed
-
- Kabbinavar F, Hurwitz HI, Fehrenbacher I, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21: 60–65 - PubMed
-
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

