Continuous infusion of the cannabinoid WIN 55,212-2 to the site of a peripheral nerve injury reduces mechanical and cold hypersensitivity
- PMID: 17375083
- PMCID: PMC2013951
- DOI: 10.1038/sj.bjp.0707210
Continuous infusion of the cannabinoid WIN 55,212-2 to the site of a peripheral nerve injury reduces mechanical and cold hypersensitivity
Abstract
Background and purpose: Cannabinoids have analgesic and anti-inflammatory properties but their use is limited by psychotropic activity at CNS receptors. Restricting cannabinoid delivery to peripheral tissues at systemically inactive doses offers a potential solution to this problem.
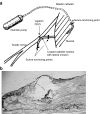
Experimental approach: WIN 55,212-2 was continuously delivered to the site of a partial ligation injury to the sciatic nerve via a perineural catheter connected to a mini-osmotic pump implanted at the time of injury. Bilateral reflex limb withdrawal behaviour was measured in adult male Wistar rats in response to mechanical and cooling stimulation of the hind paw.
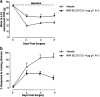
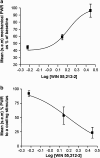
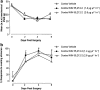
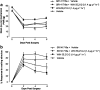
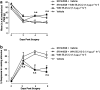
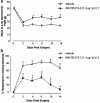
Key results: Compared with vehicle treatment, WIN 55,212-2 (1.4 microg microl(-1) hr(-1)) reduced hypersensitivity to stimuli applied to the injured limb at 2, 4 and 6 days after injury. The effects of WIN 55,212-2 (0.6-2.8 microg microl(-1) hr(-1)) were dose-dependent. Estimated EC(50) values for reduction in mean responses to mechanical and cooling stimulation (day 4 post-surgery) were 1.55 (95% C.I, [1.11-2.16]) microg microl(-1) hr(-1) and 1.52 (95% C.I, [1.07-2.18]) microg microl(-1) hr(-1), respectively. When delivered to the contralateral side to injury, WIN 55,212-2 (1.4 or 2.8 microg microl(-1) hr(-1)) did not significantly affect nerve injury-associated hypersensitivity. Co-perineural application of a CB(1) receptor antagonist SR141716a and WIN 55,212-2 prevented the effects of WIN 55,212-2 on hypersensitivity. Co-application of CB(2) receptor antagonist SR144528 reversed WIN 55,212-2's effect on mechanical hypersensitivity on day 2 only.
Conclusions and implications: These data support a peripheral antihyperalgesic effect of WIN 55,212-2 when delivered directly to the site of a nerve injury at systemically inactive doses.
Figures
References
-
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurones by activating both the cannabinoid 1 receptor and the vannilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. - PubMed
-
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. - PubMed
-
- Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabinoids. Lancet Neurol. 2003;2:291–298. - PubMed
-
- Bell MR, D'Ambra TE, Kumar V, Eissenstat MA, Herrmann JL, Wetzel JR, et al. Anti-nociceptive (aminoalkyl)indoles. J Med Chem. 1991;34:1099–1110. - PubMed
-
- Beltramo M, Bernardini R, Bertorelli M, Campanella E, Nicolussi S, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

