Role of heat shock protein 70 in induction of stress fiber formation in rat arterial endothelial cells in response to stretch stress
- PMID: 17375204
- PMCID: PMC1828078
- DOI: 10.1267/ahc.06011
Role of heat shock protein 70 in induction of stress fiber formation in rat arterial endothelial cells in response to stretch stress
Abstract
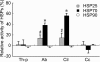
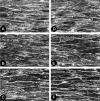
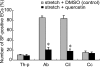
We investigated the mechanism by which endothelial cells (ECs) resist various forms of physical stress using an experimental system consisting of rat arterial EC sheets. Formation of actin stress fibers (SFs) and expression of endothelial heat-shock stress proteins (HSPs) in response to mechanical stretch stress were assessed by immunofluorescence microscopy. Stretch stimulation increased expression of HSPs 25 and 70, but not that of HSP 90. Treatment with SB203580, a p38 MAP kinase inhibitor that acts upstream of the HSP 25 activation cascade, or with geldanamycin, an inhibitor of HSP 90, had no effect on the SF formation response to mechanical stretch stress. In contrast, treatment with quercetin, an HSP 70 inhibitor, inhibited both upregulation of endothelial HSP 70 and formation of SFs in response to tensile stress. In addition, treatment of stretched ECs with cytochalasin D, which disrupts SF formation, did not adversely affect stretch-induced upregulation of endothelial HSP 70. Our data suggest that endothelial HSP 70 plays an important role in inducing SF formation in response to tensile stress.
Figures





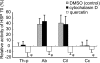
References
-
- Azuma N., Akasaka N., Kito H., Ikeda M., Gahtan V., Sasajima T., Sumpio B. E. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am. J. Physiol. 2001;280:H189–197. - PubMed
-
- Burridge K., Fath K., Kelly T., Nuckollos G. C., Turner C. Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Ann. Rev. Cell Biol. 1988;4:487–525. - PubMed
-
- Chan Y. C., Shukla N., Abdus-Samee M., Berwanger C. S., Stanford J., Singh M., Mansfield A. O., Stansby G. Anti-heat-shock protein 70 kDa antibodies in vascular patients. Eur. J. Vasc. Endovasc. Surg. 1999;18:381–385. - PubMed
-
- Chien S. S., Li S., Shyy Y. J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. - PubMed
-
- Craig E. A., Weissman J. S., Horwich A. L. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. - PubMed

