Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells
- PMID: 17376076
- PMCID: PMC2844904
- DOI: 10.1111/j.1365-2958.2007.05630.x
Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells
Abstract
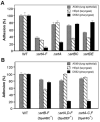
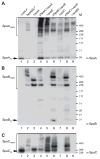
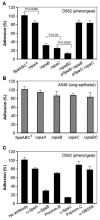
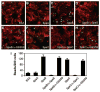
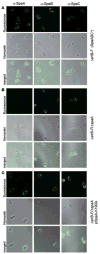
Adherence to host tissues mediated by pili is pivotal in the establishment of infection by many bacterial pathogens. Corynebacterium diphtheriae assembles on its surface three distinct pilus structures. The function and the mechanism of how various pili mediate adherence, however, have remained poorly understood. Here we show that the SpaA-type pilus is sufficient for the specific adherence of corynebacteria to human pharyngeal epithelial cells. The deletion of the spaA gene, which encodes the major pilin forming the pilus shaft, abolishes pilus assembly but not adherence to pharyngeal cells. In contrast, adherence is greatly diminished when either minor pilin SpaB or SpaC is absent. Antibodies directed against either SpaB or SpaC block bacterial adherence. Consistent with a direct role of the minor pilins, latex beads coated with SpaB or SpaC protein bind specifically to pharyngeal cells. Therefore, tissue tropism of corynebacteria for pharyngeal cells is governed by specific minor pilins. Importantly, immunoelectron microscopy and immunofluorescence studies reveal clusters of minor pilins that are anchored to cell surface in the absence of a pilus shaft. Thus, the minor pilins may also be cell wall anchored in addition to their incorporation into pilus structures that could facilitate tight binding to host cells during bacterial infection.
Figures




References
-
- Ankri S, Reyes O, Leblon G. Electrotransformation of highly DNA-restrictive corynebacteria with synthetic DNA. Plasmid. 1996;35:62–66. - PubMed
-
- Braun L, Ohayon H, Cossart P. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

