Cardiac GPCRs: GPCR signaling in healthy and failing hearts
- PMID: 17376402
- PMCID: PMC1892229
- DOI: 10.1016/j.bbamem.2007.02.010
Cardiac GPCRs: GPCR signaling in healthy and failing hearts
Abstract
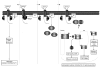
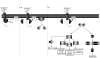
G protein-coupled receptors (GPCRs) are widely implicated in human heart disease, making them an important target for cardiac drug therapy. The most commonly studied and clinically targeted cardiac GPCRs include the adrenergic, angiotensin, endothelin, and adenosine receptors. Treatment options focusing on the complex and integrated signaling pathways of these GPCRs are critical for the understanding and amelioration of heart disease. The focus of this review is to highlight the most commonly studied and clinically targeted cardiac GPCRs, placing emphasis on their common signaling components implicated in cardiac disease.
Figures

References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Nat Rev Mol Cell Biol. 2002;3:639–50. - PubMed
-
- Hopkins AL, Groom CR. Nat Rev Drug Discov. 2002;1:727–30. - PubMed
-
- Tang CM, Insel PA. Trends Cardiovasc Med. 2004;14:94–9. - PubMed
-
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Circulation. 2005;112:e154–235. - PubMed
-
- Gether U, Kobilka BK. J Biol Chem. 1998;273:17979–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

