Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness
- PMID: 17376432
- PMCID: PMC2100382
- DOI: 10.1016/j.yexcr.2007.02.009
Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness
Abstract
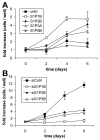
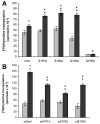
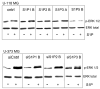
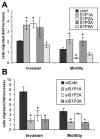
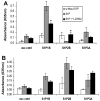
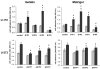
Sphingosine-1-phosphate (S1P) is a bioactive lipid that signals through a family of five G-protein-coupled receptors, termed S1P(1-5). S1P stimulates growth and invasiveness of glioma cells, and high expression levels of the enzyme that forms S1P, sphingosine kinase-1, correlate with short survival of glioma patients. In this study we examined the mechanism of S1P stimulation of glioma cell proliferation and invasion by either overexpressing or knocking down, by RNA interference, S1P receptor expression in glioma cell lines. S1P(1), S1P(2) and S1P(3) all contribute positively to S1P-stimulated glioma cell proliferation, with S1P(1) being the major contributor. Stimulation of glioma cell proliferation by these receptors correlated with activation of ERK MAP kinase. S1P(5) blocks glioma cell proliferation, and inhibits ERK activation. S1P(1) and S1P(3) enhance glioma cell migration and invasion. S1P(2) inhibits migration through Rho activation, Rho kinase signaling and stress fiber formation, but unexpectedly, enhances glioma cell invasiveness by stimulating cell adhesion. S1P(2) also potently enhances expression of the matricellular protein CCN1/Cyr61, which has been implicated in tumor cell adhesion, and invasion as well as tumor angiogenesis. A neutralizing antibody to CCN1 blocked S1P(2)-stimulated glioma invasion. Thus, while S1P(2) decreases glioma cell motility, it may enhance invasion through induction of proteins that modulate glioma cell interaction with the extracellular matrix.
Figures
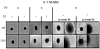



References
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
-
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. - PubMed
-
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. - PubMed
-
- Lefranc F, Sadeghi N, Camby I, Metens T, Dewitte O, Kiss R. Present and potential future issues in glioblastoma treatment. Expert Rev Anticancer Ther. 2006;6:719–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

