Structural basis of aquaporin inhibition by mercury
- PMID: 17376483
- PMCID: PMC3535476
- DOI: 10.1016/j.jmb.2007.02.070
Structural basis of aquaporin inhibition by mercury
Abstract
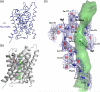
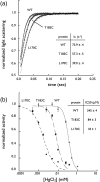
The aquaporin family of channels was defined based on the inhibition of water transport by mercurial compounds. Despite the important role of mercurials, little is known about the structural changes involved upon mercury binding leading to channel inhibition. To elucidate the mechanism we designed a mutant, T183C, of aquaporin Z (AqpZ) patterned after the known mercury-sensitive site of aquaporin 1 (AQP1) and determined the X-ray crystal structures of the unbound and mercury blocked states. Superposition of the two structures shows no conformational rearrangement upon mercury binding. In the blocked structure, there are two mercury sites, one bound to Cys183 and occluding the pore, and a second, also bound to the same cysteine but found buried in an interstitial cavity. To test the mechanism of blockade we designed a different mutant, L170C, to produce a more effective mercury block at the pore site. In a dose-response inhibition study, this mutant was 20 times more sensitive to mercury than wild-type AqpZ and four times more sensitive than T183C. The X-ray structure of L170C shows four mercury atoms at, or near, the pore site defined in the T183C structure and no structural change upon mercury binding. Thus, we elucidate a steric inhibition mechanism for this important class of channels by mercury.
Figures




References
-
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–58. - PubMed
-
- Hille B. Ion channels of excitable membranes. 3rd edit Sinauer; Sunderland, Mass: 2001.
-
- Macey RI. Transport of water and urea in red blood cells. Am J Physiol. 1984;246:C195–203. - PubMed
-
- Zeidel ML, Ambudkar SV, Smith BL, Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31:7436–40. - PubMed
-
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

