The maternally localized RNA fatvg is required for cortical rotation and germ cell formation
- PMID: 17376659
- PMCID: PMC2435194
- DOI: 10.1016/j.mod.2007.02.001
The maternally localized RNA fatvg is required for cortical rotation and germ cell formation
Abstract
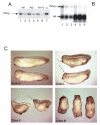
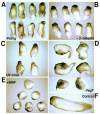
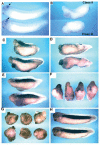
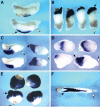
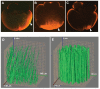
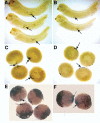
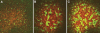
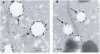
Fatvg is a localized maternal transcript that translocates to the vegetal cortex of Xenopus laevis oocytes through both the METRO and Late RNA localization pathways. It is a member of a gene family that functions in vesicular trafficking. Depletion of the maternal store of fatvg mRNA results in a dual phenotype in which embryos are ventralized and also lack primordial germ cells. This complex fatvg loss of function phenotype is the result of stabilization of the dorsalizing factor beta-catenin at the vegetal pole and the inability of the germ cell determinants to move to their proper locations. This is coincident with the inhibition of cortical rotation and the abnormal aggregation of the germ plasm. Fatvg protein is located at the periphery of vesicles in the oocyte and embryo, supporting its proposed role in vesicular trafficking in the embryo. These results point to a common fundamental mechanism that is regulated by fatvg through which germ cell determinants and dorsalizing factors segregate during early development.
Figures
Similar articles
-
The vegetally localized mRNA fatvg is associated with the germ plasm in the early embryo and is later expressed in the fat body.Mech Dev. 2001 Jan;100(1):137-40. doi: 10.1016/s0925-4773(00)00517-7. Mech Dev. 2001. PMID: 11118900
-
fatvg encodes a new localized RNA that uses a 25-nucleotide element (FVLE1) to localize to the vegetal cortex of Xenopus oocytes.Development. 1999 Nov;126(22):4943-53. doi: 10.1242/dev.126.22.4943. Development. 1999. PMID: 10529413
-
Centroid, a novel putative DEAD-box RNA helicase maternal mRNA, is localized in the mitochondrial cloud in Xenopus laevis oocytes.Int J Dev Biol. 2007;51(8):701-6. doi: 10.1387/ijdb.072293mk. Int J Dev Biol. 2007. PMID: 17939116
-
RNA localization and germ cell determination in Xenopus.Int Rev Cytol. 2001;203:63-91. doi: 10.1016/s0074-7696(01)03004-2. Int Rev Cytol. 2001. PMID: 11131528 Review.
-
Organisation of Xenopus oocyte and egg cortices.Microsc Res Tech. 1999 Mar 15;44(6):415-29. doi: 10.1002/(SICI)1097-0029(19990315)44:6<415::AID-JEMT3>3.0.CO;2-4. Microsc Res Tech. 1999. PMID: 10211675 Review.
Cited by
-
Characterization and functional roles of paternal RNAs in 2-4 cell bovine embryos.Sci Rep. 2019 Dec 30;9(1):20347. doi: 10.1038/s41598-019-55868-3. Sci Rep. 2019. PMID: 31889064 Free PMC article.
-
Primordial Germ Cell Development in the Poeciliid, Gambusia holbrooki, Reveals Shared Features Between Lecithotrophs and Matrotrophs.Front Cell Dev Biol. 2022 Mar 1;10:793498. doi: 10.3389/fcell.2022.793498. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35300414 Free PMC article.
-
Hecate/Grip2a acts to reorganize the cytoskeleton in the symmetry-breaking event of embryonic axis induction.PLoS Genet. 2014 Jun 26;10(6):e1004422. doi: 10.1371/journal.pgen.1004422. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24967891 Free PMC article.
-
Vegetally localized Xenopus trim36 regulates cortical rotation and dorsal axis formation.Development. 2009 Sep;136(18):3057-65. doi: 10.1242/dev.036855. Epub 2009 Aug 12. Development. 2009. PMID: 19675128 Free PMC article.
-
The polarization of the G-protein activated potassium channel GIRK5 to the vegetal pole of Xenopus laevis oocytes is driven by a di-leucine motif.PLoS One. 2013 May 22;8(5):e64096. doi: 10.1371/journal.pone.0064096. Print 2013. PLoS One. 2013. PMID: 23717539 Free PMC article.
References
-
- al-Mukhtar KA, Webb AC. An ultrastructural study of primordial germ cells, oogonia and early oocytes in Xenopus laevis. J Embryol Exp Morphol. 1971;26:195–217. - PubMed
-
- Basyuk E, Galli T, Mougel M, Blanchard J-M, Sitbon M, Bertrand E. Retroviral Genomic RNAs Are Transported to the Plasma Membrane by Endosomal Vesicles. Developmental Cell. 2003;5:161–174. - PubMed
-
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg 1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. - PubMed
-
- Blackler AW. Contribution to the study of germ-cells in the Anura. J Embryol Exp Morph. 1958;6:491–503. - PubMed
-
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

