Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions
- PMID: 17376862
- PMCID: PMC1865640
- DOI: 10.1128/CVI.00435-06
Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions
Abstract
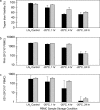
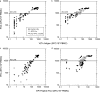
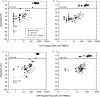
The enzyme-linked immunospot (ELISPOT) assay is a powerful tool for measuring antigen-specific cellular immune responses. The ability to use frozen peripheral blood mononuclear cells (PBMC) facilitates testing samples in multicenter clinical trials; however, unreliable ELISPOT responses may result if samples are not handled properly. Exposure of frozen PBMC to suboptimal storage temperature (-20 degrees C) or repeated cycling between more optimal storage temperatures (less than -130 degrees C and -70 degrees C) reduced the quality of frozen PBMC, as assessed by cell viability and functional ELISPOT response measures. Cell viability as assessed by trypan blue dye exclusion was reduced, and the percentage of apoptotic cells, as determined by the Guava Nexin assay, was significantly increased after these events. The functional gamma interferon ELISPOT responses to phytohemagglutinin (PHA) mitogen, a CD4 T-cell-specific antigen (varicella-zoster virus), and a CD8 T-cell-specific antigen (pool containing known cytomegalovirus, Epstein-Barr virus, and influenza virus peptides) were all significantly reduced after suboptimal storage events. However, for a given suboptimal storage event, the magnitude of the reduction varied between individuals and even among aliquots within an individual bleed, indicating the need for sample-specific acceptance criteria (AC). The percent viable or percent apoptotic cells after thaw, as well as the functional ELISPOT response to PHA, were all effective when applied with limits as AC for separating samples damaged during storage from valid control samples. Although all three AC measures could be effectively applied, the apoptosis AC limit applied was best for separating samples that could respond to antigenic stimulation from samples that could not effectively respond.
Figures




References
-
- Abrahamsen, J. F., A. M. Bakken, and O. Bruserud. 2002. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 42:1573-1580. - PubMed
-
- Arlen, P., K.-Y. Tsang, J. L. Marshall, A. Chen, S. M. Steinberg, D. Poole, P. Horan Hand, J. Schlom, and J. M. Hamilton. 2000. The use of a rapid ELISPOT assay to analyze peptide-specific immune response in carcinoma patients to peptide versus recombinant poxvirus vaccines. Cancer Immunol. Immunother. 49:517-529. - PMC - PubMed
-
- Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. - PubMed
-
- Bailey, T., S. Stark, A. Grant, C. Hartnett, M. Tsang, and A. Kalyuzhny. 2002. A multidonor ELISPOT study of IL-1B, IL-2, IL-4, IL-6, IL-13, IFN-γ and TNF-α release by cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 270:171-182. - PubMed
-
- Baust, J. M., R. van Buskirk, and J. G. Baust. 2000. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev. Dev. Biol. Animal 36:262-270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

