Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1
- PMID: 17376917
- PMCID: PMC1900291
- DOI: 10.1128/JVI.02074-06
Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1
Abstract
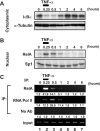
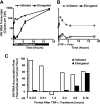
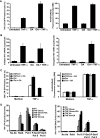
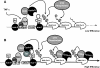
Cells harboring infectious, but transcriptionally latent, human immunodeficiency virus type 1 (HIV-1) proviruses currently pose an insurmountable barrier to viral eradication in infected patients. To better understand the molecular basis for HIV-1 latency, we used the J-Lat model of postintegration HIV-1 latency to assess the kinetic relationship between the induction of NF-kappaB and the activation of latent HIV-1 gene expression. Chromatin immunoprecipitation analyses revealed an oscillating pattern of RelA recruitment to the HIV-1 long terminal repeat (LTR) during continuous tumor necrosis factor alpha (TNF-alpha) stimulation. RNA polymerase II (Pol II) recruitment to the HIV-1 LTR closely mirrored RelA binding. Transient stimulation of cells with TNF-alpha for 15 min induced only a single round of RelA and RNA Pol II binding and failed to induce robust expression of latent HIV-1. Efficient formation of elongated HIV-1 transcripts required sustained induction by NF-kappaB, which promoted de novo synthesis of Tat. Cyclin-dependent kinase 9 (CDK9) and serine-2-phosphorylated RNA Pol II were rapidly recruited to the HIV-1 LTR after NF-kappaB induction; however, these elongating polymerase complexes were progressively dephosphorylated in the absence of Tat. Okadaic acid promoted sustained serine-2 phosphorylation of the C-terminal domain of RNA Pol II and stimulated efficient transcriptional elongation and HIV-1 expression in the absence of Tat. These findings underscore important differences between NF-kappaB and Tat stimulation of RNA Pol II elongation. While NF-kappaB binding to the HIV-1 LTR induces serial waves of efficient RNA Pol II initiation, elongation is impaired by the action of an okadaic acid-sensitive phosphatase that dephosphorylates the C-terminal domain of RNA Pol II. Conversely, the action of this phosphatase is overcome in the presence of Tat, promoting very efficient RNA Pol II elongation.
Figures




Similar articles
-
NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation.EMBO J. 2006 Jan 11;25(1):139-49. doi: 10.1038/sj.emboj.7600900. Epub 2005 Dec 1. EMBO J. 2006. PMID: 16319923 Free PMC article.
-
Semen Exosomes Promote Transcriptional Silencing of HIV-1 by Disrupting NF-κB/Sp1/Tat Circuitry.J Virol. 2018 Oct 12;92(21):e00731-18. doi: 10.1128/JVI.00731-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111566 Free PMC article.
-
Transdominant mutants of I kappa B alpha block Tat-tumor necrosis factor synergistic activation of human immunodeficiency virus type 1 gene expression and virus multiplication.J Virol. 1996 Sep;70(9):5777-85. doi: 10.1128/JVI.70.9.5777-5785.1996. J Virol. 1996. PMID: 8709193 Free PMC article.
-
Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator.IUBMB Life. 2001 Mar;51(3):175-81. doi: 10.1080/152165401753544241. IUBMB Life. 2001. PMID: 11547919 Review.
-
Tackling Tat.J Mol Biol. 1999 Oct 22;293(2):235-54. doi: 10.1006/jmbi.1999.3060. J Mol Biol. 1999. PMID: 10550206 Review.
Cited by
-
HIV Infection and TLR Signalling in the Liver.Gastroenterol Res Pract. 2012;2012:473925. doi: 10.1155/2012/473925. Epub 2012 Feb 22. Gastroenterol Res Pract. 2012. PMID: 22474436 Free PMC article.
-
A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs.PLoS One. 2012;7(1):e30176. doi: 10.1371/journal.pone.0030176. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291913 Free PMC article.
-
Carbon Monoxide Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by the Cyclic GMP/Protein Kinase G and NF-κB Signaling Pathway.J Virol. 2016 Dec 16;91(1):e01866-16. doi: 10.1128/JVI.01866-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795439 Free PMC article.
-
Host Cell Redox Alterations Promote Latent HIV-1 Reactivation through Atypical Transcription Factor Cooperativity.Viruses. 2022 Oct 18;14(10):2288. doi: 10.3390/v14102288. Viruses. 2022. PMID: 36298843 Free PMC article.
-
Hit-and-run stimulation: a novel concept to reactivate latent HIV-1 infection without cytokine gene induction.J Virol. 2010 Sep;84(17):8712-20. doi: 10.1128/JVI.00523-10. Epub 2010 Jun 10. J Virol. 2010. PMID: 20538859 Free PMC article.
References
-
- Barboric, M., R. M. Nissen, S. Kanazawa, N. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8:327-337. - PubMed
-
- Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. - PubMed
-
- Bohnlein, E., J. W. Lowenthal, M. Siekevitz, D. W. Ballard, B. R. Franza, and W. C. Greene. 1988. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell 53:827-836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

