Structure of immature West Nile virus
- PMID: 17376919
- PMCID: PMC1900247
- DOI: 10.1128/JVI.00037-07
Structure of immature West Nile virus
Abstract
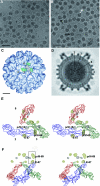
The structure of immature West Nile virus particles, propagated in the presence of ammonium chloride to block virus maturation in the low-pH environment of the trans-Golgi network, was determined by cryo-electron microscopy (cryo-EM). The structure of these particles was similar to that of immature West Nile virus particles found as a minor component of mature virus samples (naturally occurring immature particles [NOIPs]). The structures of mature infectious flaviviruses are radically different from those of the immature particles. The similarity of the ammonium chloride-treated particles and NOIPs suggests either that the NOIPs have not undergone any conformational change during maturation or that the conformational change is reversible. Comparison with the cryo-EM reconstruction of immature dengue virus established the locations of the N-linked glycosylation sites of these viruses, verifying the interpretation of the reconstructions of the immature flaviviruses.
Figures

Similar articles
-
Structure of the immature dengue virus at low pH primes proteolytic maturation.Science. 2008 Mar 28;319(5871):1834-7. doi: 10.1126/science.1153264. Science. 2008. PMID: 18369148
-
Membrane curvature in flaviviruses.J Struct Biol. 2013 Jul;183(1):86-94. doi: 10.1016/j.jsb.2013.04.005. Epub 2013 Apr 18. J Struct Biol. 2013. PMID: 23602814 Free PMC article.
-
West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody.Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12400-4. doi: 10.1073/pnas.0603488103. Epub 2006 Aug 8. Proc Natl Acad Sci U S A. 2006. PMID: 16895988 Free PMC article.
-
Structure of complex viruses and virus-infected cells by electron cryo tomography.Curr Opin Microbiol. 2006 Aug;9(4):437-42. doi: 10.1016/j.mib.2006.06.016. Epub 2006 Jul 10. Curr Opin Microbiol. 2006. PMID: 16829161 Review.
-
Manifestations of West Nile neuroinvasive disease.Rev Med Virol. 2006 Jul-Aug;16(4):209-24. doi: 10.1002/rmv.501. Rev Med Virol. 2006. PMID: 16906589 Review.
Cited by
-
A novel inhibitor of dengue virus replication that targets the capsid protein.Antimicrob Agents Chemother. 2013 Jan;57(1):15-25. doi: 10.1128/AAC.01429-12. Epub 2012 Oct 15. Antimicrob Agents Chemother. 2013. PMID: 23070172 Free PMC article.
-
Profiling of viral proteins expressed from the genomic RNA of Japanese encephalitis virus using a panel of 15 region-specific polyclonal rabbit antisera: implications for viral gene expression.PLoS One. 2015 Apr 27;10(4):e0124318. doi: 10.1371/journal.pone.0124318. eCollection 2015. PLoS One. 2015. PMID: 25915765 Free PMC article.
-
Early Events in Japanese Encephalitis Virus Infection: Viral Entry.Pathogens. 2018 Aug 13;7(3):68. doi: 10.3390/pathogens7030068. Pathogens. 2018. PMID: 30104482 Free PMC article. Review.
-
Inhibition of West Nile Virus Multiplication in Cell Culture by Anti-Parkinsonian Drugs.Front Microbiol. 2016 Mar 7;7:296. doi: 10.3389/fmicb.2016.00296. eCollection 2016. Front Microbiol. 2016. PMID: 27014219 Free PMC article.
-
Zika virus: An emerging flavivirus.J Microbiol. 2017 Mar;55(3):204-219. doi: 10.1007/s12275-017-7063-6. Epub 2017 Feb 28. J Microbiol. 2017. PMID: 28243937 Review.
References
-
- Baker, T. S., and R. H. Cheng. 1996. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J. Struct. Biol. 116:120-130. - PubMed
-
- Caspar, D. L. D., and A. Klug. 1962. Physical principles in the construction of regular viruses. Cold Spring Harbor Symp. Quant. Biol. 27:1-24. - PubMed
-
- Demaurex, N., W. Furuya, S. D'Souza, J. S. Bonifacino, and S. Grinstein. 1998. Mechanism of acidification of the trans-Golgi network (TGN). J. Biol. Chem. 273:2044-2051. - PubMed
-
- Frank, J., M. Radermacher, P. Penczek, J. Zhu, Y. Li, M. Ladjadj, and A. Leith. 1996. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116:190-199. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

