Dysregulation of HER2/HER3 signaling axis in Epstein-Barr virus-infected breast carcinoma cells
- PMID: 17376931
- PMCID: PMC1900270
- DOI: 10.1128/JVI.00076-07
Dysregulation of HER2/HER3 signaling axis in Epstein-Barr virus-infected breast carcinoma cells
Abstract
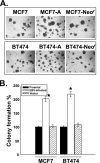
The role of Epstein-Barr virus (EBV) in the pathogenesis of breast cancer has been of long-standing interest to the field. Breast epithelial cells can be infected by EBV through direct contact with EBV-bearing lymphoblastoid cells, and EBV infection has recently been shown to confer breast cancer cells an increased resistance to chemotherapeutic drugs. In this study, we established EBV-infected breast cancer MCF7 and BT474 cells and demonstrated that EBV infection promotes tumorigenic activity of breast cancer cells. Firstly, we showed that the EBV-infected MCF7-A and BT474-A cells exhibited increased anchorage-independent growth in soft agar. The increased colony formation capacity in soft agar was associated with increased expression and activation of HER2/HER3 signaling cascades, as evidenced by the findings that the treatment of HER2 antibody trastuzumab (Herceptin), phosphatidylinositol 3-kinase inhibitor, or MEK inhibitor completely abolished the tumorigenic capacity. In the EBV-infected breast cancer cells, the expression of EBV latency genes including EBNA1, EBER1, and BARF0 was detected. We next showed that BARF0 alone was sufficient to efficiently up-regulate HER2/HER3 expression and promoted tumorigenic activity in MCF7 and BT474 cells by the use of both overexpression and small interfering RNA knock-down. Collectively, we demonstrated that EBV-encoded BARF0 promotes the tumorigenic activity of breast cancer cells through activation of HER2/HER3 signaling cascades.
Figures






Similar articles
-
Localization of Epstein-Barr virus to infiltrating lymphocytes in breast carcinomas and not malignant cells.Exp Mol Pathol. 2011 Aug;91(1):466-70. doi: 10.1016/j.yexmp.2011.04.018. Epub 2011 May 6. Exp Mol Pathol. 2011. PMID: 21600202
-
Tea polyphenol (-)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling.J Agric Food Chem. 2007 Jun 27;55(13):5030-7. doi: 10.1021/jf070316r. Epub 2007 Jun 1. J Agric Food Chem. 2007. PMID: 17539658
-
HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines.J Immunol. 2012 Mar 1;188(5):2136-45. doi: 10.4049/jimmunol.1102237. Epub 2012 Feb 1. J Immunol. 2012. PMID: 22301547
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Reactivation of Epstein-Barr virus from latency.Rev Med Virol. 2005 May-Jun;15(3):149-56. doi: 10.1002/rmv.456. Rev Med Virol. 2005. PMID: 15546128 Review.
Cited by
-
The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation.J Biol Chem. 2008 Jun 27;283(26):18393-401. doi: 10.1074/jbc.M801329200. Epub 2008 Apr 22. J Biol Chem. 2008. PMID: 18434311 Free PMC article.
-
Lack of Association Between Epstein-Barr Virus and Mammary Tumours in Dogs.J Vet Res. 2018 Dec 10;62(3):309-315. doi: 10.2478/jvetres-2018-0045. eCollection 2018 Sep. J Vet Res. 2018. PMID: 30584610 Free PMC article.
-
Recent Advances on the Possible Neuroprotective Activities of Epstein-Barr Virus Oncogene BARF1 Protein in Chronic Inflammatory Disorders of Central Nervous System.Curr Neuropharmacol. 2010 Sep;8(3):268-75. doi: 10.2174/157015910792246191. Curr Neuropharmacol. 2010. PMID: 21358976 Free PMC article.
-
Epstein-Barr virus infection and clinical outcome in breast cancer patients correlate with immune cell TNF-α/IFN-γ response.BMC Cancer. 2014 Sep 11;14:665. doi: 10.1186/1471-2407-14-665. BMC Cancer. 2014. PMID: 25213133 Free PMC article.
-
Peptide Vaccines as Therapeutic and Prophylactic Agents for Female-Specific Cancers: The Current Landscape.Pharmaceuticals (Basel). 2023 Jul 24;16(7):1054. doi: 10.3390/ph16071054. Pharmaceuticals (Basel). 2023. PMID: 37513965 Free PMC article. Review.
References
-
- Alimandi, M., A. Romano, M. C. Curia, R. Muraro, P. Fedi, S. A. Aaronson, P. P. Di Fiore, and M. H. Kraus. 1995. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 10:1813-1821. - PubMed
-
- Arbach, H., V. Viglasky, F. Lefeu, J. M. Guinebretiere, V. Ramirez, N. Bride, N. Boualaga, T. Bauchet, J. P. Peyrat, M. C. Mathieu, S. Mourah, M. P. Podgorniak, J. M. Seignerin, K. Takada, and I. Joab. 2006. Epstein-Barr virus (EBV) genome and expression in breast cancer tissue: effect of EBV infection of breast cancer cells on resistance to paclitaxel (Taxol). J. Virol. 80:845-853. - PMC - PubMed
-
- Baumforth, K. R., J. R. Flavell, G. M. Reynolds, G. Davies, T. R. Pettit, W. Wei, S. Morgan, T. Stankovic, Y. Kishi, H. Arai, M. Nowakova, G. Pratt, J. Aoki, M. J. Wakelam, L. S. Young, and P. G. Murray. 2005. Induction of autotaxin by the Epstein-Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood 106:2138-2146. - PubMed
-
- Bobrow, L. G., R. R. Millis, L. C. Happerfield, and W. J. Gullick. 1997. c-erbB-3 protein expression in ductal carcinoma in situ of the breast. Eur. J. Cancer 33:1846-1850. - PubMed
-
- Bonnet, M., J. M. Guinebretiere, E. Kremmer, V. Grunewald, E. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

