Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia
- PMID: 17376970
- PMCID: PMC6672474
- DOI: 10.1523/JNEUROSCI.4854-06.2007
Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia
Abstract
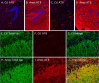
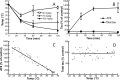
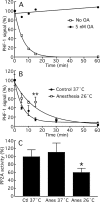
Postoperative cognitive dysfunction, confusion, and delirium are common after general anesthesia in the elderly, with symptoms persisting for months or years in some patients. Even middle-aged patients are likely to have postoperative cognitive dysfunction for months after surgery, and Alzheimer's disease (AD) patients appear to be particularly at risk of deterioration after anesthesia. Several investigators have thus examined whether general anesthesia is associated with AD, with some studies suggesting that exposure to anesthetics may increase the risk of AD. However, little is known on the biochemical consequences of anesthesia on pathogenic pathways in vivo. Here, we investigated the effect of anesthesia on tau phosphorylation and amyloid precursor protein (APP) metabolism in mouse brain. We found that, regardless of the anesthetic used, anesthesia induced rapid and massive hyperphosphorylation of tau, rapid and prolonged hypothermia, inhibition of Ser/Thr PP2A (protein phosphatase 2A), but no changes in APP metabolism or Abeta (beta-amyloid peptide) accumulation. Reestablishing normothermia during anesthesia completely rescued tau phosphorylation to normal levels. Our results indicate that changes in tau phosphorylation were not a result of anesthesia per se, but a consequence of anesthesia-induced hypothermia, which led to inhibition of phosphatase activity and subsequent hyperphosphorylation of tau. These findings call for careful monitoring of core temperature during anesthesia in laboratory animals to avoid artifactual elevation of protein phosphorylation. Furthermore, a thorough examination of the effect of anesthesia-induced hypothermia on the risk and progression of AD is warranted.
Figures




References
-
- Amaducci LA, Fratiglioni L, Rocca WA, Fieschi C, Livrea P, Pedone D, Bracco L, Lippi A, Gandolfo C, Bino G, Prencipe M, Bonatti ML, Girotti F, Carella F, Tavola B, Ferla S, Lenzi GL, Carolei A, Gambi A, Grigoletto F, et al. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of an Italian population. Neurology. 1986;36:922–931. - PubMed
-
- Ancelin ML, de Roquefeuil G, Ledesert B, Bonnel F, Cheminal JC, Ritchie K. Exposure to anaesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry. 2001;178:360–366. - PubMed
-
- Avila J. Tau phosphorylation and aggregation in Alzheimer's disease pathology. FEBS Lett. 2006;580:2922–2927. - PubMed
-
- Avila J, Diaz-Nido J. Tangling with hypothermia. Nat Med. 2004;10:460–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
