Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation
- PMID: 17376973
- PMCID: PMC6672463
- DOI: 10.1523/JNEUROSCI.0054-07.2007
Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation
Abstract
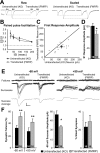
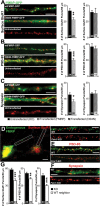
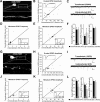
Fragile X syndrome, as well as other forms of mental retardation and autism, is associated with altered dendritic spine number and structure. Fragile X syndrome is caused by loss-of-function mutations in Fragile X mental retardation protein (FMRP), an RNA-binding protein that regulates protein synthesis in vivo. It is unknown whether FMRP plays a direct, cell-autonomous role in regulation of synapse number, function, or maturation. Here, we report that acute postsynaptic expression of FMRP in Fmr1 knock-out (KO) neurons results in a decrease in the number of functional and structural synapses without an effect on their synaptic strength or maturational state. Similarly, neurons endogenously expressing FMRP (wild-type) have fewer synapses than neighboring Fmr1 KO neurons. An intact K homology domain 2 (KH2) RNA-binding domain and dephosphorylation of FMRP at S500 were required for the effects of FMRP on synapse number, indicating that FMRP interaction with RNA and translating polyribosomes leads to synapse loss.
Figures



References
-
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. - PubMed
-
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006 - PubMed
-
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
-
- Braun K, Segal M. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex. 2000;10:1045–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
