Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear
- PMID: 17376975
- PMCID: PMC6672483
- DOI: 10.1523/JNEUROSCI.5151-06.2007
Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear
Abstract
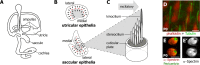
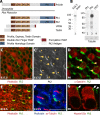
Vestibular hair cells have a distinct planar cell polarity (PCP) manifest in the morphology of their stereocilia bundles and the asymmetric localization of their kinocilia. In the utricle and saccule the hair cells are arranged in an orderly array about an abrupt line of reversal that separates fields of cells with opposite polarity. We report that the putative PCP protein Prickle-like 2 (Pk2) is distributed in crescents on the medial sides of vestibular epithelial cells before the morphological polarization of hair cells. Despite the presence of a line of polarity reversal, crescent position is not altered between hair cells of opposite polarity. Frizzled 6 (Fz6), a second PCP protein, is distributed opposite Pk2 along the lateral side of vestibular support cells. Similar to Pk2, the subcellular localization of Fz6 does not differ between cells located on opposite sides of the line of reversal. In addition, in Looptail/Van Gogh-like2 mutant mice Pk2 is distributed asymmetrically at embryonic day 14.5 (E14.5), but this localization is not coordinated between adjacent cells, and the crescents subsequently are lost by E18.5. Together, these results support the idea that a conserved PCP complex acts before stereocilia bundle development to provide an underlying polarity to all cells in the vestibular epithelia and that cells on either side of the line of reversal are programmed to direct the kinocilium in opposite directions with respect to the polarity axis defined by PCP protein distribution.
Figures



References
-
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. - PubMed
-
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. - PubMed
-
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localized and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. - PubMed
-
- Bekman E, Henrique D. Embryonic expression of three mouse genes with homology to the Drosophila melanogaster prickle gene. Mech Dev. 2002;119(Suppl 1):S77–S81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
