Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone
- PMID: 17376977
- PMCID: PMC6672487
- DOI: 10.1523/JNEUROSCI.4969-06.2007
Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone
Abstract
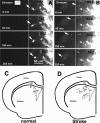
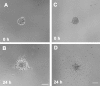
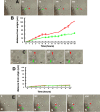
Ischemic stroke induces neurogenesis in the subventricular zone (SVZ), and newly generated neurons in the SVZ migrate toward the ischemic boundary. However, the characteristics of migrating SVZ cells have not been investigated after stroke. Using time-lapse imaging in both SVZ cells and organotypic brain slice cultures, we measured the dynamics of SVZ cell division and migration of adult rats subjected to stroke. In normal brain slices, SVZ cells primarily migrated dorsally and ventrally along the lateral ventricular surface. However, in stroke brain slices, SVZ cells migrated laterally toward the striatal ischemic boundary. Cultured stroke-derived SVZ cells exhibited a significant (p < 0.01) increase in the migration distance (212 +/- 21 microm) compared with the nonstroke-derived SVZ cells (97 +/- 12 microm). Migrating stroke-derived SVZ cells spent significantly (p = 0.01) less time in cytokinesis (0.63 +/- 0.04 h) compared with the time (1.09 +/- 0.09 h) for nonstroke-derived SVZ cells. Newborn cells with a single leading process exhibited fast migration (7.2 +/- 0.8 microm/h), and cells with multiple processes showed stationary migration (3.6 +/- 0.8 microm/h). Stroke SVZ daughter cells further divided during their migration. The morphology of doublecortin (DCX)-positive cells in fixed brain sections resembled those observed in cultured newborn cells, and the DCX-positive cells proliferated in the ischemic striatum. Collectively, the present study suggests that stroke promotes cytokinesis of migrating neuroblasts, and these cells migrate toward the ischemic striatum with distinct migratory behaviors and retain the capacity for cell division during migration.
Figures


References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Feng Y, Walsh CA. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci. 2001;2:408–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
