Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells
- PMID: 17376979
- PMCID: PMC6672455
- DOI: 10.1523/JNEUROSCI.3965-06.2007
Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells
Abstract
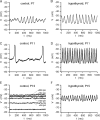
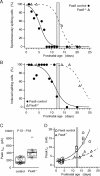
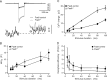
Thyroid hormone (TH) is essential for the development of hearing. Lack of TH in a critical developmental period from embryonic day 17 to postnatal day 12 (P12) in rats and mice leads to morphological and functional deficits in the organ of Corti and the auditory pathway. We investigated the effects of TH on inner hair cells (IHCs) using patch-clamp recordings, capacitance measurements, and immunocytochemistry in hypothyroid rats and athyroid Pax8-/- mice. Spontaneous and evoked Ca2+ action potentials (APs) were present in control IHCs from P3-P11 rats and vanished in parallel with the expression of a rapidly activating Ca2+- and voltage-activated K+ (BK) conductance. IHCs of hypothyroid rats and athyroid Pax8-/- mice displayed APs until the end of the third postnatal week because of threefold elevated Ca2+ currents and missing expression of BK currents. After the fourth postnatal week, some IHCs showed BK currents whereas adjacent IHCs did not, demonstrated by electrophysiology and immunocytochemistry. To test whether the prolonged spiking activity during TH deficiency may be transmitted at IHC synapses, capacitance measurements were performed in parallel to analysis of otoferlin expression, a protein thought to play an essential role in exocytosis of IHCs. Strikingly, otoferlin was absent from IHCs of hypothyroid rats but not of Pax8-/- mice, although both cell types showed exocytosis with an efficiency typical for immature IHCs. These results demonstrate for the first time a TH-dependent control of IHC spiking activity before the onset of hearing attributable to effects of TH on Ca2+ and BK channels. Moreover, they question an indispensable role of otoferlin for exocytosis in IHCs.
Figures







Similar articles
-
Maturation of ribbon synapses in hair cells is driven by thyroid hormone.J Neurosci. 2007 Mar 21;27(12):3163-73. doi: 10.1523/JNEUROSCI.3974-06.2007. J Neurosci. 2007. PMID: 17376978 Free PMC article.
-
Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells.J Physiol. 2003 Apr 15;548(Pt 2):383-400. doi: 10.1113/jphysiol.2002.034801. Epub 2003 Feb 14. J Physiol. 2003. PMID: 12588897 Free PMC article.
-
Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels.J Neurosci. 2003 Mar 15;23(6):2141-9. doi: 10.1523/JNEUROSCI.23-06-02141.2003. J Neurosci. 2003. PMID: 12657673 Free PMC article.
-
How to build an inner hair cell: challenges for regeneration.Hear Res. 2007 May;227(1-2):3-10. doi: 10.1016/j.heares.2006.12.005. Epub 2006 Dec 16. Hear Res. 2007. PMID: 17258412 Review.
-
Kölliker's organ and the development of spontaneous activity in the auditory system: implications for hearing dysfunction.Biomed Res Int. 2014;2014:367939. doi: 10.1155/2014/367939. Epub 2014 Aug 20. Biomed Res Int. 2014. PMID: 25210710 Free PMC article. Review.
Cited by
-
Talking back: Development of the olivocochlear efferent system.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e324. doi: 10.1002/wdev.324. Epub 2018 Jun 26. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29944783 Free PMC article. Review.
-
Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants.J Neurosci. 2009 Jan 28;29(4):1212-23. doi: 10.1523/JNEUROSCI.4957-08.2009. J Neurosci. 2009. PMID: 19176829 Free PMC article.
-
Developmental regulation of spontaneous activity in the Mammalian cochlea.J Neurosci. 2010 Jan 27;30(4):1539-50. doi: 10.1523/JNEUROSCI.3875-09.2010. J Neurosci. 2010. PMID: 20107081 Free PMC article.
-
Fast Ca2+ Transients of Inner Hair Cells Arise Coupled and Uncoupled to Ca2+ Waves of Inner Supporting Cells in the Developing Mouse Cochlea.Front Mol Neurosci. 2018 Jul 30;11:264. doi: 10.3389/fnmol.2018.00264. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30104958 Free PMC article.
-
A modifier gene alleviates hypothyroidism-induced hearing impairment in Pou1f1dw dwarf mice.Genetics. 2011 Oct;189(2):665-73. doi: 10.1534/genetics.111.130633. Epub 2011 Aug 11. Genetics. 2011. PMID: 21840860 Free PMC article.
References
-
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni MA, Koby M, Weintraub BD. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1996;81:2768–2772. - PubMed
-
- Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW. Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol. 2004;9:88–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
