Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation
- PMID: 17376983
- PMCID: PMC4922751
- DOI: 10.1523/JNEUROSCI.5265-06.2007
Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation
Abstract
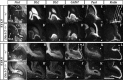
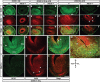
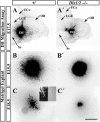
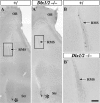
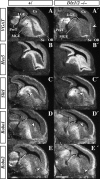
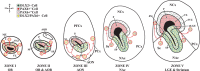
Olfactory bulb interneuron development is a complex multistep process that involves cell specification in the ventral telencephalon, tangential migration into the olfactory bulb, and local neuronal maturation. Although several transcription factors have been implicated in this process, how or when they act remains to be elucidated. Here we explore the mechanisms that result in olfactory bulb interneuron defects in Dlx1&2-/- (distal-less homeobox 1 and 2) and Mash1-/- (mammalian achaete-schute homolog 1) mutants. We provide evidence that Dlx1&2 and Mash1 regulate parallel molecular pathways that are required for the generation of these cells, thereby providing new insights into the mechanisms underlying olfactory bulb development. The analysis also defined distinct anatomical zones related to olfactory bulb development. Finally we show that Dlx1&2 are required for promoting tangential migration to the olfactory bulb, potentially via regulating the expression of ErbB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4), Robo2 (roundabout homolog 2), Slit1 (slit homolog 1), and PK2 (prokineticin 2), which have all been shown to play essential roles in this migration.
Figures



Similar articles
-
GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors.J Neurosci. 2017 Sep 6;37(36):8816-8829. doi: 10.1523/JNEUROSCI.2125-16.2017. Epub 2017 Aug 8. J Neurosci. 2017. PMID: 28821666 Free PMC article.
-
The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons.Neuron. 2006 Feb 16;49(4):503-16. doi: 10.1016/j.neuron.2006.01.018. Neuron. 2006. PMID: 16476661
-
Dlx1/2 are Central and Essential Components in the Transcriptional Code for Generating Olfactory Bulb Interneurons.Cereb Cortex. 2019 Dec 17;29(11):4831-4849. doi: 10.1093/cercor/bhz018. Cereb Cortex. 2019. PMID: 30796806 Free PMC article.
-
Spatio-temporal specification of olfactory bulb interneurons.J Mol Histol. 2007 Dec;38(6):563-9. doi: 10.1007/s10735-007-9111-8. Epub 2007 Jun 22. J Mol Histol. 2007. PMID: 17588153 Review.
-
Temporal patterns of olfactory bulb interneuron neurogenesis.J Neurosci. 2008 Aug 13;28(33):8145-7. doi: 10.1523/JNEUROSCI.2321-08.2008. J Neurosci. 2008. PMID: 18701675 Free PMC article. Review. No abstract available.
Cited by
-
Neurog1 and Neurog2 control two waves of neuronal differentiation in the piriform cortex.J Neurosci. 2014 Jan 8;34(2):539-53. doi: 10.1523/JNEUROSCI.0614-13.2014. J Neurosci. 2014. PMID: 24403153 Free PMC article.
-
Decoding Cortical Glial Cell Development.Neurosci Bull. 2021 Apr;37(4):440-460. doi: 10.1007/s12264-021-00640-9. Epub 2021 Feb 19. Neurosci Bull. 2021. PMID: 33606177 Free PMC article.
-
Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon.Neural Dev. 2009 Feb 10;4:5. doi: 10.1186/1749-8104-4-5. Neural Dev. 2009. PMID: 19208224 Free PMC article.
-
The transcription factor Sp8 is required for the production of parvalbumin-expressing interneurons in the olfactory bulb.J Neurosci. 2011 Jun 8;31(23):8450-5. doi: 10.1523/JNEUROSCI.0939-11.2011. J Neurosci. 2011. PMID: 21653849 Free PMC article.
-
Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways.Cereb Cortex. 2009 Jul;19 Suppl 1(Suppl 1):i96-106. doi: 10.1093/cercor/bhp045. Epub 2009 Apr 22. Cereb Cortex. 2009. PMID: 19386638 Free PMC article.
References
-
- Anchan RM, Drake DP, Haines CF, Gerwe EA, LaMantia AS. Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J Comp Neurol. 1997;379:171–184. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–476. - PubMed
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. - PubMed
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
