Basic fibroblast growth factor modulates density of blood vessels and preserves tight junctions in organotypic cortical cultures of mice: a new in vitro model of the blood-brain barrier
- PMID: 17376986
- PMCID: PMC6672460
- DOI: 10.1523/JNEUROSCI.4033-06.2007
Basic fibroblast growth factor modulates density of blood vessels and preserves tight junctions in organotypic cortical cultures of mice: a new in vitro model of the blood-brain barrier
Abstract
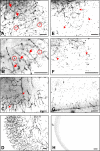
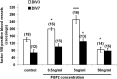
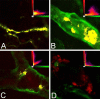
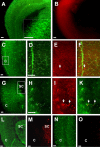
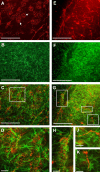
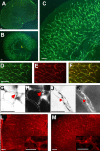
This study was performed to examine the maintenance of blood vessels in vitro in cortical organotypic slice cultures of mice with special emphasis on basic fibroblast growth factor (FGF-2), which is known to promote angiogenesis and to preserve the integrity of the blood-brain barrier. Slices of neonatal day 3 or 4 mouse brain were maintained for 3, 7, or 10 d in vitro (DIV) under standard culture conditions or in the presence of FGF-2. Immunohistochemistry for factor VIII-related antigen or laminin revealed a relative low number of blood vessels under standard conditions. In contrast, moderate FGF-2 concentrations increased the number of vessels: with 0.5 ng/ml FGF-2 it was 1.4-fold higher after DIV 3 or 1.5-fold after DIV 7 compared with controls; with 5 ng/ml it was almost doubled in both cases. With an excess of 50 ng/ml, FGF-2 vessels were reduced after DIV 3 or similar to controls after DIV 7. FGF receptor 1 was preferentially found on endothelial cells; its immunolabeling was reduced in the presence of the ligand. Cell death detected by an ethidium bromide analog or the apoptosis marker caspase-3 was barely detectable during the 10 d culture period. Immunolabeling of the tight junction proteins ZO-1 (zonula occludens protein 1), occludin, claudin-5, and claudin-3 revealed evidence for structural integrity of the blood-brain barrier in the presence of moderate FGF-2 concentrations. In conclusion, FGF-2 maintains blood vessels in vitro and preserves the composition of the tight junction. Hence, we propose FGF-2-treated organotypic cortical slices as a new tool for mechanistic studies of the blood-brain barrier.
Figures
References
-
- Anderson JM, van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. - PubMed
-
- Balda MS, Matter K. Tight junctions. J Cell Sci. 1998;111:541–547. - PubMed
-
- Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs AR, Davis TP. Protection against hypoxia-induced increase in blood–brain barrier permeability: role of tight junction proteins and NFκB. J Cell Sci. 2003;116:693–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
