Multiple translational isoforms give functional specificity to serum- and glucocorticoid-induced kinase 1
- PMID: 17377066
- PMCID: PMC1877090
- DOI: 10.1091/mbc.e06-10-0968
Multiple translational isoforms give functional specificity to serum- and glucocorticoid-induced kinase 1
Abstract
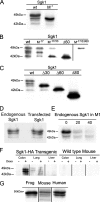
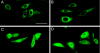
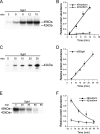
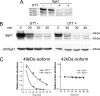
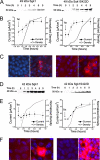
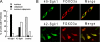
Serum- and glucocorticoid-induced kinase 1 is a ubiquitous kinase that regulates diverse processes such as ion transport and cell survival. We report that a single SGK1 mRNA produces isoforms with different N-termini owing to alternative translation initiation. The long isoforms, 49 and 47 kDa, are the most abundant, localize to the ER membrane, exhibit rapid turnover, their expression is decreased by ER stress, activate the epithelial sodium channel (ENaC) and translocate FoxO3a transcriptional factors from the nucleus to the cytoplasm. The short isoforms, 45 and 42 kDa, localize to the cytoplasm and nucleus, exhibit long half-life and phosphorylate glycogen synthase kinase-3beta. The data indicate that activation of Sgk1 in different cellular compartments is key to providing functional specificity to Sgk1 signaling pathways. We conclude that the distinct properties and functional specialization of Sgk1 given by the N-terminus confer versatility of function while maintaining the same core kinase domain.
Figures
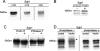
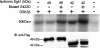
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

