Delivery of retinoid-based therapies to target tissues
- PMID: 17378589
- PMCID: PMC2562735
- DOI: 10.1021/bi7003069
Delivery of retinoid-based therapies to target tissues
Abstract
Through its various metabolites, vitamin A controls essential physiological functions. Both naturally occurring metabolites and novel retinoid analogues have shown effectiveness in many clinical settings that include skin diseases and cancer, and in animal models of human conditions affecting vision. In this review, we analyze several potential retinoid-based therapies from the point of view of drug metabolism and transport to target tissues. We focus on the endogenous factors that affect the absorption, transport, and metabolism of retinoids by taking into account data obtained from the analysis of animal models that lack the enzymes or proteins involved in the storage and absorption of retinoids. We also discuss findings of toxicity associated with retinoids in an effort to improve the outcome of retinoid-based therapies. In this context, we review evidence that esterification of retinol and retinol-based drugs within target tissues provides one of the most efficient means to improve the absorption and to reduce the toxicity associated with pharmacological doses of retinoids. Future retinoid-based therapeutic strategies could involve targeted delivery mechanisms leading to lower toxicity and improved effectiveness of retinoids.
Figures
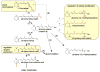

References
-
- Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. - PubMed
-
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. - PubMed
-
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. - PubMed
-
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. - PubMed
-
- Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991;254:1654–1656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

