Defective B cell ontogeny and immune response in human complement receptor 2 (CR2, CD21) transgenic mice is partially recovered in the absence of C3
- PMID: 17379312
- PMCID: PMC1948027
- DOI: 10.1016/j.molimm.2007.02.011
Defective B cell ontogeny and immune response in human complement receptor 2 (CR2, CD21) transgenic mice is partially recovered in the absence of C3
Abstract
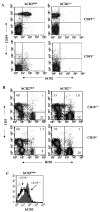
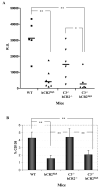
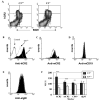
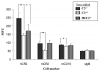
Mice prematurely expressing human CR2 (hCR2) in the B cell lineage have a defective B cell ontogeny and immune response. Our recent analysis of this phenotype suggested that signaling through hCR2 and presumably mouse CD19 on the B cell surface, during bone marrow development, could result in the observed changes in B cell function in these mice. To test this hypothesis, we back crossed hCR2(high) transgenic mice onto the CD19(-/-) background. CD19(-/-)hCR2(high) mice were found to possess even fewer mature B cells than their CD19(+/+)hCR2(high) littermates, demonstrating that loss of CD19 exacerbated the effects elicited through hCR2. This data suggests that CD19 provides a survival signal during B cell development in this model. Next, we examined if the removal of the main ligand for CR2, namely C3d, through back-crossing onto the C3(-/-) background could restore normal B cell development. However, we found only minor recovery in peripheral B cell numbers and no obvious change in function. This was despite a three-fold increase in the level of hCR2 expression on B cells isolated from the spleen or bone marrow of C3(-/-)hCR2(high) mice when compared with C3 sufficient littermates. These data demonstrate that hCR2 is integrated in mouse B cell signaling and that the downstream effects of hCR2 expression during early B cell development are partially but not completely due to interaction with C3 fragments and signaling through CD19 in the bone marrow environment.
Figures
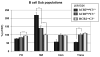

References
-
- Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–62. - PubMed
-
- Asokan R, Hua J, Young KA, Gould HJ, Hannan JP, Kraus DM, Szakonyi G, Grundy GJ, Chen XS, Crow MK, Holers VM. Characterization of human complement receptor type 2 (CR2/CD21) as a receptor for IFN-alpha: a potential role in systemic lupus erythematosus. J Immunol. 2006;177:383–94. - PubMed
-
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. - PMC - PubMed
-
- Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001;167:4172–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

