The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons
- PMID: 17379413
- PMCID: PMC2225450
- DOI: 10.1016/j.pain.2007.01.035
The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons
Abstract
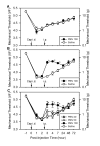
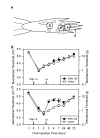
Previous findings that reactive oxygen species (ROS) are involved in neuropathic pain, mainly through spinal mechanisms, suggest that ROS may be involved in central sensitization. To investigate the possible role of ROS in central sensitization, we examined in rats the effects of ROS scavengers on capsaicin-induced secondary hyperalgesia, which is known to be mediated by central sensitization. We used two different ROS scavengers: phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL). Intradermal capsaicin injection (20 microg in 20 microl olive oil) into the hind paw produced primary and secondary hyperalgesia. A systemic administration of PBN (100mg/kg, i.p.) or TEMPOL (200mg/kg, i.p.) alleviated capsaicin-induced secondary, but not primary, hyperalgesia. Intrathecal injection of PBN (1mg inof veterinary Surgery/anesthesiology, College of veterinary Medic 50 microl saline) greatly reduced hyperalgesia, whereas intracerebroventricular or intradermal injection of PBN produced only a minor analgesic effect, suggesting that PBN takes effect mainly through the spinal cord. Electrophysiological recordings from wide dynamic range (WDR) neurons in the dorsal horn showed that intradermal capsaicin enhanced the evoked responses to peripheral stimuli; systemic PBN or TEMPOL restored the responses to normal levels. Removal of ROS thus restored the responsiveness of spinal WDR neurons to normal levels, suggesting that ROS is involved in central sensitization, at least in part by sensitizing WDR neurons.
Figures




Similar articles
-
Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice.Pain. 2008 Sep 15;138(3):514-524. doi: 10.1016/j.pain.2008.01.029. Epub 2008 Mar 28. Pain. 2008. PMID: 18375065 Free PMC article.
-
Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain.Redox Biol. 2018 Apr;14:391-397. doi: 10.1016/j.redox.2017.10.011. Epub 2017 Oct 16. Redox Biol. 2018. PMID: 29055283 Free PMC article.
-
Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain.Pain. 2007 Oct;131(3):262-271. doi: 10.1016/j.pain.2007.01.011. Epub 2007 Feb 20. Pain. 2007. PMID: 17317010 Free PMC article.
-
Hyperalgesia and sensitization of dorsal horn neurons following activation of NK-1 receptors in the rostral ventromedial medulla.J Neurophysiol. 2017 Nov 1;118(5):2727-2744. doi: 10.1152/jn.00478.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794197 Free PMC article.
-
Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release.Pain. 2011 Apr;152(4):844-852. doi: 10.1016/j.pain.2010.12.034. Epub 2011 Feb 5. Pain. 2011. PMID: 21296500 Free PMC article.
Cited by
-
Orexin-A inhibits capsaicin-induced changes in cyclooxygenase-2 and brain-derived neurotrophic factor expression in trigeminal nucleus caudalis of rats.Korean J Pain. 2018 Jul;31(3):174-182. doi: 10.3344/kjp.2018.31.3.174. Epub 2018 Jul 2. Korean J Pain. 2018. PMID: 30013731 Free PMC article.
-
Effects of Fu's subcutaneous needling on mitochondrial structure and function in rats with sciatica.Mol Pain. 2022 Apr;18:17448069221108717. doi: 10.1177/17448069221108717. Mol Pain. 2022. PMID: 35670088 Free PMC article.
-
Recent development in antihyperalgesic effect of phytochemicals: anti-inflammatory and neuro-modulatory actions.Inflamm Res. 2018 Aug;67(8):633-654. doi: 10.1007/s00011-018-1156-5. Epub 2018 May 16. Inflamm Res. 2018. PMID: 29767332 Review.
-
Intraganglionic reactive oxygen species mediate inflammatory pain and hyperalgesia through TRPA1 in the rat.Front Pain Res (Lausanne). 2023 May 30;4:1204057. doi: 10.3389/fpain.2023.1204057. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37325677 Free PMC article.
-
Nitroxidative Stress, Cell-Signaling Pathways, and Manganese Porphyrins: Therapeutic Potential in Neuropathic Pain.Int J Mol Sci. 2025 Feb 26;26(5):2050. doi: 10.3390/ijms26052050. Int J Mol Sci. 2025. PMID: 40076672 Free PMC article. Review.
References
-
- Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. - PubMed
-
- Contestabile A. Oxidative stress in neurodegeneration: mechanisms and therapeutic perspectives. Curr Top Med Chem. 2001;1:553–568. - PubMed
-
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. - PubMed
-
- Guedes RP, Bosco LD, Teixeira CM, Araújo ASR, Llesuy S, Belló-Klein A, Ribeiro MFM, Partata WA. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

