Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1
- PMID: 17379599
- PMCID: PMC2659598
- DOI: 10.1074/jbc.M701279200
Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1
Abstract
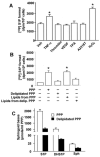
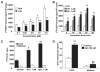
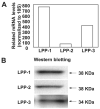
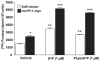
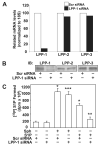
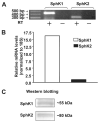
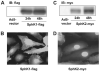
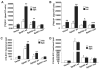
Sphingosine 1-phosphate (S1P) regulates diverse cellular functions through extracellular ligation to S1P receptors, and it also functions as an intracellular second messenger. Human pulmonary artery endothelial cells (HPAECs) effectively utilized exogenous S1P to generate intracellular S1P. We, therefore, examined the role of lipid phosphate phosphatase (LPP)-1 and sphingosine kinase1 (SphK1) in converting exogenous S1P to intracellular S1P. Exposure of (32)P-labeled HPAECs to S1P or sphingosine (Sph) increased the intracellular accumulation of [(32)P]S1P in a dose- and time-dependent manner. The S1P formed in the cells was not released into the medium. The exogenously added S1P did not stimulate the sphingomyelinase pathway; however, added [(3)H]S1P was hydrolyzed to [(3)H]Sph in HPAECs, and this was blocked by XY-14, an inhibitor of LPPs. HPAECs expressed LPP1-3, and overexpression of LPP-1 enhanced the hydrolysis of exogenous [(3)H]S1P to [(3)H]Sph and increased intracellular S1P production by 2-3-fold compared with vector control cells. Down-regulation of LPP-1 by siRNA decreased intracellular S1P production from extracellular S1P but had no effect on the phosphorylation of Sph to S1P. Knockdown of SphK1, but not SphK2, by siRNA attenuated the intracellular generation of S1P. Overexpression of wild type SphK1, but not SphK2 wild type, increased the accumulation of intracellular S1P after exposure to extracellular S1P. These studies provide the first direct evidence for a novel pathway of intracellular S1P generation. This involves the conversion of extracellular S1P to Sph by LPP-1, which facilitates Sph uptake, followed by the intracellular conversion of Sph to S1P by SphK1.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

