The effect of external sodium concentration on sodium-calcium exchange in frog olfactory receptor cells
- PMID: 17379630
- PMCID: PMC2075203
- DOI: 10.1113/jphysiol.2007.131094
The effect of external sodium concentration on sodium-calcium exchange in frog olfactory receptor cells
Abstract
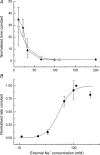
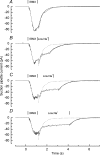
During the response of vertebrate olfactory receptor cells to stimulation, Ca(2+) enters the cilia via cyclic nucleotide-gated channels and is extruded by Na(+)-Ca(2+) exchange. The rise in Ca(2+) concentration opens a Ca(2+)-activated Cl(-) conductance which carries most of the inward receptor current. The dependence of Ca(2+) extrusion upon external Na(+) concentration was studied by using the falling phase of the Ca(2+)-activated Cl(-) current following a brief exposure to the phosphodiesterase inhibitor IBMX to monitor indirectly the decay in intraciliary Ca(2+) concentration. External Na(+) concentration was reduced by partial substitution with guanidinium, an ion which permeates the cyclic nucleotide-gated channel but does not support Na(+)-Ca(2+) exchange. The time constant describing the decay in current following IBMX stimulation was surprisingly little affected by substitution of external Na(+), being substantially retarded only when its concentration was reduced to a third or less of its normal value in Ringer solution. When the cilia were returned to Ringer solution after a period in reduced-Na(+) solution, the time constant for the final decay of current was similar to that seen when returning immediately to IBMX-free Ringer solution. This observation suggests that Ca(2+) extrusion via Na(+)-Ca(2+) exchange dominates the falling phase of the response to IBMX, which can therefore be used to assess exchanger activity. Rate constants derived from the time constants for current decay at different external Na(+) concentrations could be fitted by the Hill equation with a K(d) of 54 +/- 4 mm and Hill coefficient of 3.7 +/- 0.4. The cooperativity of the dependence upon external Na(+) concentration indicates that at least three Na(+) ions enter for each exchanger cycle, while the high affinity for external Na(+) contrasts with the photoreceptor and cardiac exchangers. The functional importance of this observation is that the relative insensitivity of the Na(+)-Ca(2+) exchanger to external Na(+) concentration allows normal response termination even following partial dilution or concentration of the olfactory mucus.
Figures




Similar articles
-
Responses to prolonged odour stimulation in frog olfactory receptor cells.J Physiol. 2001 Jul 1;534(Pt 1):179-91. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. J Physiol. 2001. PMID: 11433001 Free PMC article.
-
Electrogenic Na(+)/Ca(2+) exchange. A novel amplification step in squid olfactory transduction.J Gen Physiol. 2000 Jun;115(6):759-68. doi: 10.1085/jgp.115.6.759. J Gen Physiol. 2000. PMID: 10828249 Free PMC article.
-
Plasma membrane Ca(2+)-ATPase in the cilia of olfactory receptor neurons: possible role in Ca(2+) clearance.Eur J Neurosci. 2007 Nov;26(9):2524-31. doi: 10.1111/j.1460-9568.2007.05863.x. Epub 2007 Oct 23. Eur J Neurosci. 2007. PMID: 17970729
-
Electrogenic Na+/Ca2+-exchange of nerve and muscle cells.Prog Neurobiol. 2007 Aug;82(6):287-347. doi: 10.1016/j.pneurobio.2007.06.003. Epub 2007 Jun 21. Prog Neurobiol. 2007. PMID: 17673353 Review.
-
Cardiac sodium transport and excitation-contraction coupling.J Mol Cell Cardiol. 2013 Aug;61:11-9. doi: 10.1016/j.yjmcc.2013.06.003. Epub 2013 Jun 14. J Mol Cell Cardiol. 2013. PMID: 23774049 Review.
Cited by
-
Limits of calcium clearance by plasma membrane calcium ATPase in olfactory cilia.PLoS One. 2009;4(4):e5266. doi: 10.1371/journal.pone.0005266. Epub 2009 Apr 23. PLoS One. 2009. PMID: 19390572 Free PMC article.
-
Mechanism of olfactory masking in the sensory cilia.J Gen Physiol. 2009 Jun;133(6):583-601. doi: 10.1085/jgp.200810085. Epub 2009 May 11. J Gen Physiol. 2009. PMID: 19433623 Free PMC article.
-
Olfactory response termination involves Ca2+-ATPase in vertebrate olfactory receptor neuron cilia.J Gen Physiol. 2010 Apr;135(4):367-78. doi: 10.1085/jgp.200910337. J Gen Physiol. 2010. PMID: 20351061 Free PMC article.
-
Cyclic-nucleotide-gated cation current and Ca2+-activated Cl current elicited by odorant in vertebrate olfactory receptor neurons.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11078-11087. doi: 10.1073/pnas.1613891113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647918 Free PMC article.
-
The Na(+)/Ca(2+) exchanger NCKX4 governs termination and adaptation of the mammalian olfactory response.Nat Neurosci. 2011 Nov 6;15(1):131-7. doi: 10.1038/nn.2943. Nat Neurosci. 2011. PMID: 22057188 Free PMC article.
References
-
- Antolin S. University of Cambridge; 2006. Sodium-calcium exchange in amphibian olfactory receptor cells. PhD Thesis.
-
- Antolin S, Matthews HR. Characterisation of the electrogenic Na+-Ca2+ exchange current in frog olfactory receptor cells. J Physiol. 2004;555.P:C160.
-
- Antolin S, Matthews HR. Effect of external Na+ on Na-Ca exchange-mediated current recovery in frog ORNs. p. A37. Association for Chemoreception Sciences meeting abstract.
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

