G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signalling in mouse cerebellar Purkinje cells
- PMID: 17379632
- PMCID: PMC2075187
- DOI: 10.1113/jphysiol.2007.129866
G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signalling in mouse cerebellar Purkinje cells
Abstract
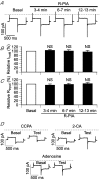
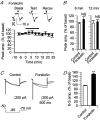
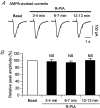
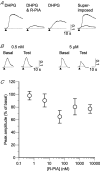
Adenosine receptors (ARs) are G protein-coupled receptors (GPCRs) mediating the neuromodulatory actions of adenosine that influence emotional, cognitive, motor, and other functions in the central nervous system (CNS). Previous studies show complex formation between ARs and metabotropic glutamate receptors (mGluRs) in heterologous systems and close colocalization of ARs and mGluRs in several central neurons. Here we explored the possibility of intimate functional interplay between G(i/o) protein-coupled A(1)-subtype AR (A1R) and type-1 mGluR (mGluR1) naturally occurring in cerebellar Purkinje cells. Using a perforated-patch voltage-clamp technique, we found that both synthetic and endogenous agonists for A1R induced continuous depression of a mGluR1-coupled inward current. A1R agonists also depressed mGluR1-coupled intracellular Ca(2+) mobilization monitored by fluorometry. A1R indeed mediated this depression because genetic depletion of A1R abolished it. Surprisingly, A1R agonist-induced depression persisted after blockade of G(i/o) protein. The depression appeared to involve neither the cAMP-protein kinase A cascade downstream of the alpha subunits of G(i/o) and G(s) proteins, nor cytoplasmic Ca(2+) that is suggested to be regulated by the beta-gamma subunit complex of G(i/o) protein. Moreover, A1R did not appear to affect G(q) protein which mediates the mGluR1-coupled responses. These findings suggest that A1R modulates mGluR1 signalling without the aid of the major G proteins. In this respect, the A1R-mediated depression of mGluR1 signalling shown here is clearly distinguished from the A1R-mediated neuronal responses described so far. These findings demonstrate a novel neuromodulatory action of adenosine in central neurons.
Figures


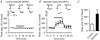



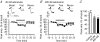


Similar articles
-
Functional cooperation of metabotropic adenosine and glutamate receptors regulates postsynaptic plasticity in the cerebellum.J Neurosci. 2013 Nov 20;33(47):18661-71. doi: 10.1523/JNEUROSCI.5567-12.2013. J Neurosci. 2013. PMID: 24259587 Free PMC article.
-
Complex formation and functional interaction between adenosine A1 receptor and type-1 metabotropic glutamate receptor.J Pharmacol Sci. 2015 Jul;128(3):125-30. doi: 10.1016/j.jphs.2015.06.002. Epub 2015 Jun 19. J Pharmacol Sci. 2015. PMID: 26154847
-
Distinct roles of Galpha(q) and Galpha11 for Purkinje cell signaling and motor behavior.J Neurosci. 2004 Jun 2;24(22):5119-30. doi: 10.1523/JNEUROSCI.4193-03.2004. J Neurosci. 2004. PMID: 15175381 Free PMC article.
-
Calcium dependence of native metabotropic glutamate receptor signaling in central neurons.Mol Neurobiol. 2004 Jun;29(3):261-70. doi: 10.1385/MN:29:3:261. Mol Neurobiol. 2004. PMID: 15181238 Review.
-
Roles of phospholipase Cbeta4 in synapse elimination and plasticity in developing and mature cerebellum.Mol Neurobiol. 2001 Feb;23(1):69-82. doi: 10.1385/MN:23:1:69. Mol Neurobiol. 2001. PMID: 11642544 Review.
Cited by
-
Interplay between metabotropic glutamate type 4 and adenosine type 1 receptors modulate synaptic transmission in the cerebellar cortex.Front Pharmacol. 2024 Aug 15;15:1406238. doi: 10.3389/fphar.2024.1406238. eCollection 2024. Front Pharmacol. 2024. PMID: 39211784 Free PMC article.
-
Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells.J Physiol. 2007 Dec 1;585(Pt 2):549-63. doi: 10.1113/jphysiol.2007.141010. Epub 2007 Oct 18. J Physiol. 2007. PMID: 17947316 Free PMC article.
-
Plasticity of burst firing induced by synergistic activation of metabotropic glutamate and acetylcholine receptors.Neuron. 2009 Jan 29;61(2):287-300. doi: 10.1016/j.neuron.2008.12.013. Neuron. 2009. PMID: 19186170 Free PMC article.
-
Type-1 metabotropic glutamate receptor signaling in cerebellar Purkinje cells in health and disease.F1000Res. 2017 Apr 4;6:416. doi: 10.12688/f1000research.10485.1. eCollection 2017. F1000Res. 2017. PMID: 28435670 Free PMC article. Review.
-
Neuroadaptations in adenosine receptor signaling following long-term ethanol exposure and withdrawal.Alcohol Clin Exp Res. 2012 Jan;36(1):4-13. doi: 10.1111/j.1530-0277.2011.01586.x. Epub 2011 Jul 18. Alcohol Clin Exp Res. 2012. PMID: 21762181 Free PMC article. Review.
References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- Balaban CD, Wurpel JND, Servers WB. A specific harmaline-evoked increase in cerebellar 5′-nucleotidase activity. Neurosci Lett. 1984;50:111–116. - PubMed
-
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. - PubMed
-
- Batchlor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

