Postnatal androgen deprivation dissociates the development of smooth muscle innervation from functional neurotransmission in mouse vas deferens
- PMID: 17379637
- PMCID: PMC2075184
- DOI: 10.1113/jphysiol.2007.128728
Postnatal androgen deprivation dissociates the development of smooth muscle innervation from functional neurotransmission in mouse vas deferens
Abstract
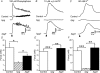
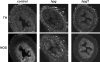
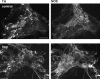
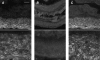
The pelvic autonomic nervous system is a target for circulating androgens in adults, with androgen exposure or deprivation affecting the structure and function of urogenital tract innervation. However, the critical period for androgen exposure to initially establish pelvic autonomic neuromuscular transmission has not been determined. We have examined the sympathetic innervation of the vas deferens in hypogonadal (hpg) mice that are deprived of androgens after birth but undergo normal prenatal sexual differentiation and remain androgen responsive throughout life. In vasa deferentia from hpg mice, purinergic excitatory junction potentials and contractions could not be elicited by electrical stimulation and P2X(1) purinoceptors could not be demonstrated by immunofluorescence. Moreover, a novel inhibitory nitrergic transmission developed. Administering testosterone to adult hpg mice restored purinergic excitatory transmission and P2X(1) purinoceptor immunofluorescence, and nitrergic inhibitory transmission was lost. Despite the deficit in excitatory neurotransmission in hpg mice, their vasa deferentia were innervated by numerous noradrenergic axons and pelvic ganglia appeared normal. In addition, noradrenergic contractions could be elicited by electrical stimulation. This study has revealed that postnatal androgen exposure has a profound effect on the development of excitatory transmission in vas deferens smooth muscle, primarily by a postjunctional action, but is not essential for development of the structural innervation of this organ. Our results also indicate that there is no postnatal critical period for androgen exposure to establish neuroeffector transmission and that postnatal androgen exposure can be delayed until adulthood, with little consequence for establishment of normal sympathetic neurotransmission.
Figures
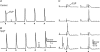
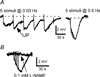
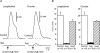
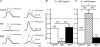

References
-
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. - PubMed
-
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology. 2002;143:4922–4933. - PubMed
-
- Brock JA, Cunnane TC, Starke K, Wardell CF. α2adrenoceptor-mediated autoinhibition of sympathetic transmitter release in guinea-pig vas deferens studied by intracellular and focal extracellualr recording of junction potentials and currents. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:45–52. - PubMed
-
- Bustamante D, Lara H, Belmar J. Changes of norepinephrine levels, tyrosine hydroxylase and dopamine-β-hydroxylase activities after castration and testosterone treatment in vas deferens of adult rats. Biol Reprod. 1989;40:541–548. - PubMed
-
- Calixto JB, Rae GA. Influence of castration of the neonatal rat on the pharmacological reactivity of the isolated vas deferens. Biol Reprod. 1981;25:481–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

