Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands
- PMID: 17379640
- PMCID: PMC2075181
- DOI: 10.1113/jphysiol.2006.127498
Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands
Abstract
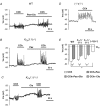
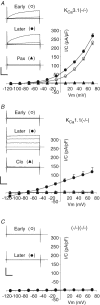
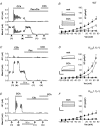
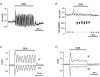
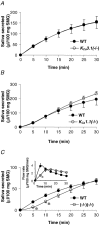
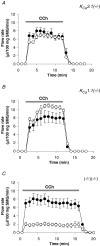
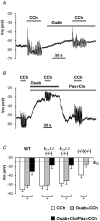
We have recently shown that the IK1 and maxi-K channels in parotid salivary gland acinar cells are encoded by the K(Ca)3.1 and K(Ca)1.1 genes, respectively, and in vivo stimulated parotid secretion is severely reduced in double-null mice. The current study tested whether submandibular acinar cell function also relies on these channels. We found that the K(+) currents in submandibular acinar cells have the biophysical and pharmacological footprints of IK1 and maxi-K channels and their molecular identities were confirmed by the loss of these currents in K(Ca)3.1- and K(Ca)1.1-null mice. Unexpectedly, the pilocarpine-stimulated in vivo fluid secretion from submandibular glands was essentially normal in double-null mice. This result and the possibility of side-effects of pilocarpine on the nervous system, led us to develop an ex vivo fluid secretion assay. Fluid secretion from the ex vivo assay was substantially (about 75%) reduced in animals with both K(+) channel genes ablated - strongly suggesting systemic complications with the in vivo assay. Additional experiments focusing on the membrane potential in isolated submandibular acinar cells revealed mechanistic details underlying fluid secretion in K(+) channel-deficient mice. The membrane potential of submandibular acinar cells from wild-type mice remained strongly hyperpolarized (-55 +/- 2 mV) relative to the Cl(-) equilibrium potential (-24 mV) during muscarinic stimulation. Similar hyperpolarizations were observed in K(Ca)3.1- and K(Ca)1.1-null mice (-51 +/- 3 and -48 +/- 3 mV, respectively), consistent with the normal fluid secretion produced ex vivo. In contrast, acinar cells from double K(Ca)3.1/K(Ca)1.1-null mice were only slightly hyperpolarized (-35 +/- 2 mV) also consistent with the ex vivo (but not in vivo) results. Finally, we found that the modest hyperpolarization of cells from the double-null mice was maintained by the electrogenic Na(+),K(+)-ATPase.
Figures

References
-
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. - PubMed
-
- Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. - PubMed
-
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. - PubMed
-
- Cecanho R, Anaya M, Renzi A, Menani JV, De Luca LA., Jr Sympathetic mediation of salivation induced by intracerebroventricular pilocarpine in rats. J Auton Nerv Syst. 1999;76:9–14. - PubMed
-
- Cook DI, Van Lennep EW, Roberts ML, Young JA. Secretion by the Major Salivary Glands. New York: Raven Press; 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

