New photoreactive tRNA derivatives for probing the peptidyl transferase center of the ribosome
- PMID: 17379815
- PMCID: PMC1852821
- DOI: 10.1261/rna.425907
New photoreactive tRNA derivatives for probing the peptidyl transferase center of the ribosome
Abstract
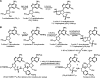
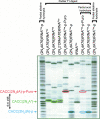
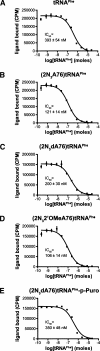
Three new photoreactive tRNA derivatives have been synthesized for use as probes of the peptidyl transferase center of the ribosome. In two of these derivatives, the 3' adenosine of yeast tRNA(Phe) has been replaced by either 2-azidodeoxyadenosine or 2-azido-2'-O-methyl adenosine, while in a third the 3'-terminal 2-azidodeoxyadenosine of the tRNA is joined to puromycin via a phosphoramidate linkage to generate a photoreactive transition-state analog. All three derivatives bind to the P site of 70S ribosomes with affinities similar to that of unmodified tRNA(Phe) and can be cross-linked to components of the 50S ribosomal subunit by irradiation with near-UV light. Characteristic differences in the cross-linking patterns suggest that these tRNA derivatives can be used to follow subtle changes in the position of the tRNA relative to the components of the peptidyl transferase center.
Figures



Similar articles
-
Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex.Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232-6. doi: 10.1073/pnas.86.14.5232. Proc Natl Acad Sci U S A. 1989. PMID: 2664777 Free PMC article.
-
Peptidyl transferase and beyond.Biochem Cell Biol. 1995 Nov-Dec;73(11-12):1041-7. doi: 10.1139/o95-111. Biochem Cell Biol. 1995. PMID: 8722019 Review.
-
Peptidyl transferase antibiotics perturb the relative positioning of the 3'-terminal adenosine of P/P'-site-bound tRNA and 23S rRNA in the ribosome.RNA. 1999 Aug;5(8):1003-13. doi: 10.1017/s1355838299990568. RNA. 1999. PMID: 10445875 Free PMC article.
-
Photochemical cross-linking of yeast tRNA(Phe) containing 8-azidoadenosine at positions 73 and 76 to the Escherichia coli ribosome.Biochemistry. 1988 Oct 18;27(21):8114-21. doi: 10.1021/bi00421a021. Biochemistry. 1988. PMID: 3069129
-
The ribosome as a versatile catalyst: reactions at the peptidyl transferase center.Curr Opin Struct Biol. 2013 Aug;23(4):595-602. doi: 10.1016/j.sbi.2013.04.012. Epub 2013 May 24. Curr Opin Struct Biol. 2013. PMID: 23711800 Review.
Cited by
-
Synthesis of Peptidyl-tRNA Mimics for Structural Biology Applications.Acc Chem Res. 2023 Oct 3;56(19):2713-2725. doi: 10.1021/acs.accounts.3c00412. Epub 2023 Sep 20. Acc Chem Res. 2023. PMID: 37728742 Free PMC article. Review.
References
-
- Agmon, I., Amit, I., Auerbach, T., Bashan, A., Baram, D., Bartels, H., Berisio, R., Greenberg, I., Harms, J., Hansen, H.A.S., et al. Ribosomal crystallography: A flexible nucleotide anchoring tRNA translocation, facilitates peptide-bond formation, chirality discrimination, and antibiotics synergism. FEBS Lett. 2004;567:20–26. - PubMed
-
- Ban, N., Nissen, P., Hansen, J., Moore, P.B., Steitz, T.A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science. 2000;289:905–920. - PubMed
-
- Barrio, J.R., Barrio, M.C., Leonard, N.J., England, T.E., Uhlenbeck, O.C. Synthesis of modified nucleoside 3′,5′-bisphosphates and their incorporation into oligoribonucleotides with T4 RNA ligase. Biochemistry. 1978;17:2077–2081. - PubMed
-
- Bashan, A., Agmon, I., Zarivach, R., Schluenzen, F., Harms, J., Berisio, R., Bartels, H., Franceschi, F., Auerbach, T., Hansen, H.A.S., et al. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell. 2003;11:91–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
